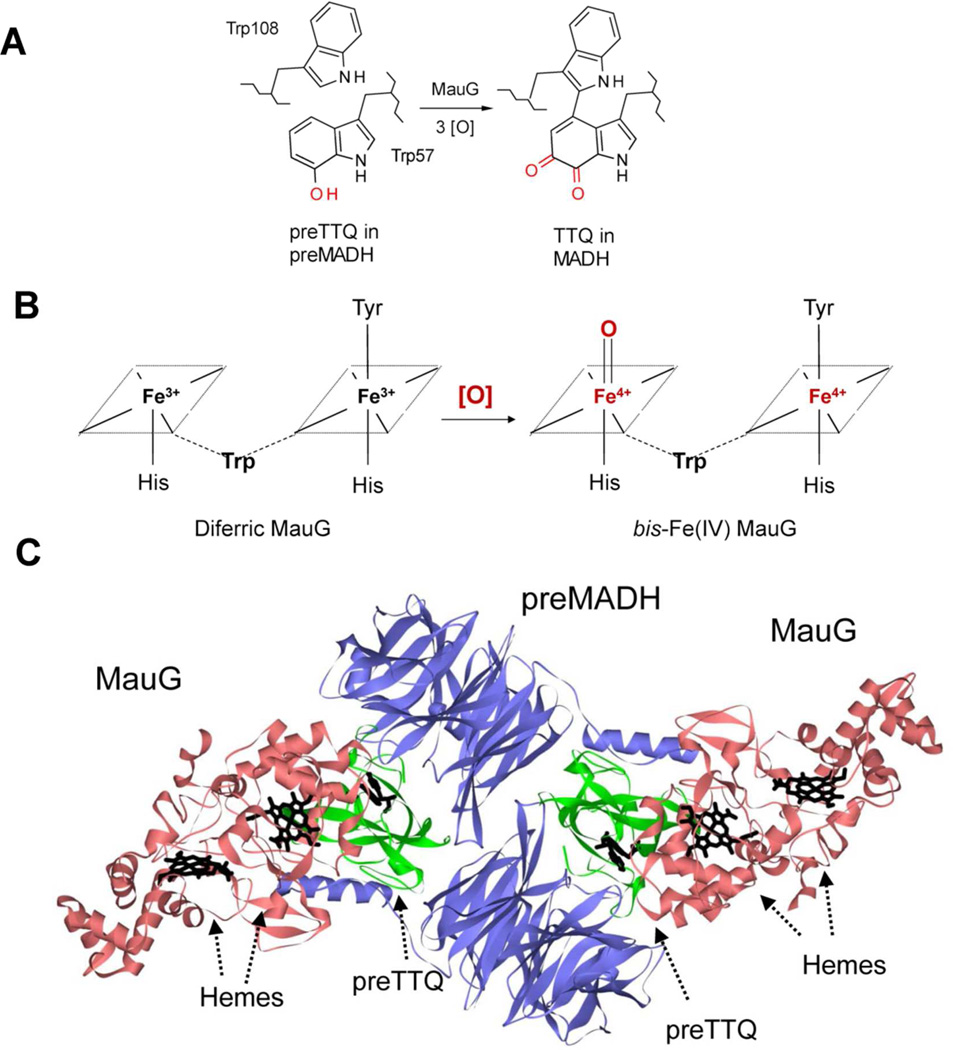
Figure 1.
A. MauG catalyzes the conversion of monohydroxylated βTrp57 and βTrp108 of preMADH to TTQ. [O] is oxidation equivalents. B. The bis-Fe(IV) state of MauG is formed by reaction of [O] with diferric MauG. The residues which form the axial ligands of the hemes and an intervening Trp residue which is believed to facilitate communication between the hemes are indicated. C. The crystal structure of the MauG-preMADH complex (PDB ID: 3L4M) (24) is shown with MauGs colored red; preMADH α subunits colored blue, and preMADH β subunits colored green. The hemes of MauG and βTrp108 and mono-hydroxylated βTrp57 of preMADH are drawn in a stick representation and colored black.