Fig 1.
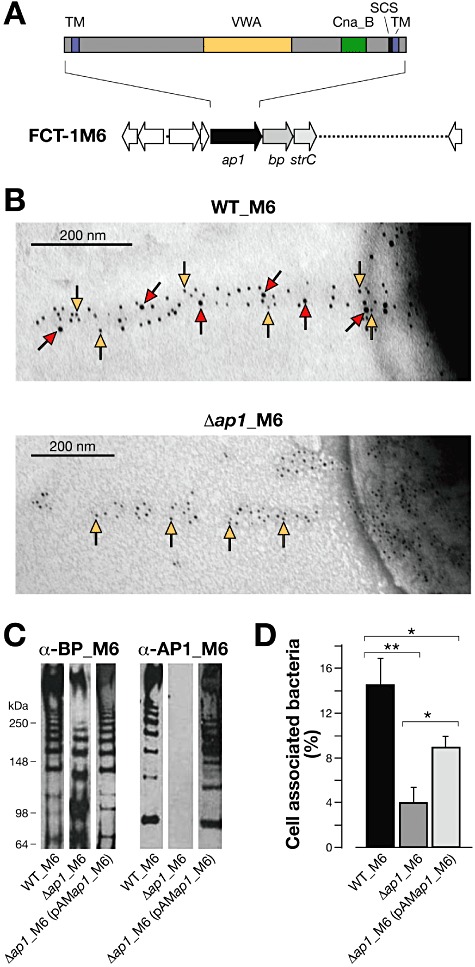
Structural organization and adhesive properties of the ancillary protein AP1_M6 of FCT-1 pili. A. Schematic representation of the FCT-1 pilus island genetic organization and of the structural domains of AP1_M6, including the hydrophobic transmembranes (TM), the Von-Willebrand factor type A domain (VWA), the Cna_B domain and the sortase cleavage site (SCS). B. Immunoelectron microscopy image taken at 30× magnification after double immunogold labelling of FCT-1 M6 pili from wild type (WT_M6) and its isogenic Δap1_M6 derivative with rabbit polyclonal anti-rBP_M6 and mouse anti-rAP1_M6 antibodies, followed by gold-labelled anti-rabbit IgG (particle size 5 nm, yellow arrows) and anti-mouse IgG antibodies (10 nm, red arrows). C. Immunoblot of cell wall fractions of WT_M6, Δap1_M6 and the complemented strain Δap1_M6(pAMap1_M6). Total protein amounts of the different extracts loaded on the gels were normalized using antibodies to protein Spy0269. Transferred extracts were incubated with specific mouse sera raised against rBP_M6 and rAP1_M6 proteins. D. Binding of WT_M6, Δap1_M6 and Δap1_M6(pAMap1_M6) to A549 cells (moi 100:1) after 30 min of incubation. Histograms represent the percentage of cell-associated versus total bacteria. Results are presented as the mean and standard deviation values of three independent experiments run in triplicate wells. Significant differences between strains calculated by Student's t-test are shown by asterisks **P < 0.01, *P < 0.05.
