Abstract
A large proportion of human tumors show deregulated expression of a variety of proteins that play a crucial role in the execution of the apoptotic program. Survivin belongs to the family of inhibitor of apoptosis proteins which were originally identified in baculoviruses. Ectopic expression of survivin conveys resistance to apoptosis to a variety of stimuli, and survivin is one of the most abundantly overexpressed genes in human tumors such as breast cancer. In this study we examined the expression of survivin protein in a series of T4 breast cancers to identify any correlation with long-term patient outcomes. Moreover, we investigated the hypothesis of a possible association between p53 and survivin as a factor further complicating the outcome. Archival specimens from 53 T4 breast cancer patients were included in the study and treated for the immunohistochemical localization of survivin and p53 using the streptavidin-biotin alkaline phosphatase method. The immunoreactivity was evaluated semiquantitatively according to the percentage of cells stained. Forty percent of tumors were positive for survivin. Statistical analysis revealed that survivin expression negatively influenced the 5- and 10-year disease-free and overall patient survival. In multivariate analysis, survivin expression was a significant independent prognostic indicator of worse outcome in overall survival [hazard ratio (HR)=2.61]. Our results showed that survivin is associated with a worse prognosis in patients with T4 breast cancer, and remarkably its prognostic relevance is maintained even long-term. Notably, p53 (HR=3.2) seems to negatively enhance the effect of survivin on survival.
Keywords: T4 breast cancer, prognosis, survivin
Introduction
Breast cancer is the most frequent tumor and the leading cause of cancer-related death among the female population worldwide (1). Histopathological factors such as the size of the primary tumor, differentiation grade, the Ki-67 protein, the expression of estrogens (ER) and progesterone (PgR) receptors or human epidermal growth factor receptor 2 (HER2) and lymph node metastasis are associated with tumor prognosis (2). Identification of mechanisms underlying tumor cell invasion may contribute to develop new therapies that can arrest local invasion and metastatic spread of the disease.
Survivin, also called baculoviral inhibitor of apoptosis repeat-containing 5 or BIRC5, is a 16.5-kDa protein which in humans is encoded by the BIRC5 gene (3,4). Survivin is a member of the inhibitor of apoptosis family that serves to inhibit caspase activation therefore leading to negative regulation of apoptosis or programmed cell death. Besides, it is known that survivin localizes to the mitotic spindle by interaction with tubulin during mitosis and may play an important role in regulating mitosis (5). Survivin is undetectable in terminally differentiated adult tissues, but becomes notably expressed in the most common human tumors, including stomach, colorectal, lung, breast, pancreatic and prostate cancers (6,7).
Survivin expression can be deregulated in cancer by several mechanisms, including amplification of the survivin locus on chromosome 17q25, demethylation of survivin exons, increased promoter activity and increased upstream signaling in the phosphatidylinositol 3-kinase or mitogen-activated protein kinase pathways. Additionally, the upregulation of survivin expression in cancer cells seems to be independent of the cell cycle, suggesting an increase in its antiapoptotic role compared with normal cells, in which its mitotic regulation functions may be predominant (8,9).
High survivin expression in the primary tumor, in many cancer types, is almost invariably associated with a poor prognosis for the patient. In breast cancer patients, however, the association of survivin with prognosis is ambiguous, since previous studies have reported it to be either irrelevant (10), or associated with poor (11) or good prognosis (12).
In this study, we tested the hypothesis that survivin expression in patients with T4 breast cancer treated with primary chemotherapy correlates with long-term outcomes. Moreover, due to the conflicting data existing on the prognostic effect of the tumor-suppressor protein p53 in breast cancer (13,14), we also investigated the hypothesis of a possible association between p53 and survivin as a factor further complicating patient outcome.
Materials and methods
Patients
This retrospective study included 53 consecutive breast cancer patients with clinical stage T4 as assessed by physical examination and mammography, confirmed via core needle biopsy. Patients were enrolled between 1992 and 2001. The median follow-up was 125 months (range, 70–182 months). All 53 patients received a multimodality treatment including primary chemotherapy, surgery, radiation therapy (RT), adjuvant chemotherapy and hormone therapy if indicated.
The median age of the patients was 50 years (range, 32–67 years). Regarding the pathological characteristics, 15 patients (28%) were diagnosed with inflammatory breast carcinoma (T4d); 38 (72%) were non-inflammatory (T4abc). Twenty-eight patients (53%) were ER-positive and 25 (47%) were ER-negative; 17 patients (32%) were PgR-positive and 36 (68%) PgR-negative; 24 patients (45%) were both ER- and PgR-negative and 16 (30%) both ER- and PgR-positive; 12 patients (23%) were ER-positive PgR-negative; 10 patients (19%) were HER2-positive and 43 (81%) HER2-negative; 18 patients (34%) were HER2-, ER- and PgR-negative (triple negative; TN), and 35 (66%) non-TN. Seventeen patients (32%) were Ki-67-positive and 27 (51%) Ki-67-negative; Ki-67 was not determined in 9 cases (17%). Baseline patient and tumor characteristics are summarized in Table I.
Table I.
Patient and tumor characteristics.
| Frequency (n) | % | |
|---|---|---|
| Age | ||
| ≤50 | 23 | 43 |
| >50 | 30 | 57 |
| Tumor stage | ||
| T4abc | 38 | 72 |
| T4d | 15 | 28 |
| Axillary nodes | ||
| cLN0 | 3 | 6 |
| cLN+ | 50 | 94 |
| Hormone receptor status | ||
| ER+/ER− | 28/25 | 53/47 |
| PgR+/PgR− | 17/36 | 32/68 |
| HER2 status | ||
| HER2+ | 10 | 19 |
| HER2− | 43 | 81 |
| Proliferative index | ||
| Ki-67+ | 17 | 32 |
| Ki-67− | 27 | 51 |
| Ki-67 unknown | 9 | 17 |
| Grading | ||
| G2 | 38 | 72 |
| G3 | 15 | 28 |
| Survivin expression | ||
| survivin+ | 21 | 40 |
| survivin− | 32 | 60 |
| p53 expression | ||
| p53+ | 13 | 24 |
| p53− | 40 | 76 |
| survivin+ and p53+ | 11 | 21 |
| survivin− and p53− | 30 | 79 |
ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Pathological assessment
ER and PgR status was assessed by standard immunohistochemical (IHC) analysis. Nuclear staining of ≥10% was considered positive. Hormone receptor (HR)+ was indicated as ER+ and PgR+, and HR− was indicated as ER− and PgR−. HER2 status was assessed by IHC or by fluorescence in situ hybridization (FISH) in breast cancer tissue. HER2-positive tumors were defined as 3+ on the IHC test. HER2-negative tumors were defined as 0 or 1+ on the IHC test; IHC 2+ required the FISH test.
Response assessment
The clinical measurement of the response to neoadjuvant therapy was defined according to the International Union Against Cancer (UICC) criteria (15) as: complete response (CR), a total resolution of the breast tumor and axillary adenopathy based on clinical and instrumental examinations; partial response (PR), a ≥50% reduction of the product of the two largest perpendicular dimensions of the breast mass and axillary adenopathy; minor response (MR), a <50% reduction of the product of the two largest perpendicular dimensions of the breast mass and axillary adenopathy; no change in clinical status (NC); and progressive disease (PD).
Pathological complete response was defined as the absence of residual invasive disease in both the breast and the axilla. Gross invasive residual disease in breast tissue or the presence of cancer-positive lymph nodes in the axilla were defined as <pCR (2). Major pathological response in breast tissue was defined as no more than 2 cm of residual disease (pT0 plus pT1) (16).
Treatment plan
All patients were treated with primary chemotherapy with anthracyline-containing regimens such as FEC (5-fluorouracil, epirubicin, cyclophosphamide) or PEV (cisplatin, epirubicin, vinorelbine).
After completing the neoadjuvant chemotherapy, 3–4 weeks after the last dose of treatment, patients underwent surgery consisting of modified radical mastectomy or breastconserving surgery. Postoperative adjuvant chemotherapy consisted of six cycles of intravenous cyclophosphamide, methotrexate and fluorouracil (CMF). The adjuvant treatment usually was initiated 3–4 weeks after surgery. Locoregional RT was performed during the fourth course of CMF.
After completing the adjuvant chemotherapy, patients with hormone receptor-positive tumors, if postmenopausal, received tamoxifen for 5 years alone.
Clinical evaluations were performed every 3 months for 2 years and every 6 months thereafter. Instrumental examinations (e.g., mammography, liver ultrasound, chest X-ray, bone scan and echocardiogram) were performed every 6 months for the first 2 years and every 12 months thereafter for ≥5 years.
Immunohistochemical staining
This study was based on an analysis of formalin-fixed, paraffin-embedded archival samples. Serial microtome sections (6- to 7-μm thick) were treated for the immunohistochemical staining of survivin and p53, using the streptavidin-biotin alkaline phosphatase method. Water-bath, heating-based antigen retrieval was performed by immersion in 10 mM citrate buffer solution (pH 6.0) at 95°C for 40 min. After gradual cooling for 20 min, the sections were treated for 45 min with 10% normal goat or normal horse serum in PBS to block nonspecific binding. Rabbit polyclonal antibody to recombinant human survivin protein (1:2000; Novus Biologicals, Littleton, CO, USA), and mouse monoclonal antibody to human p53 protein (1:50, clone DO-7; Dako, Glostrup, Denmark) were used as primary antisera. Biotinylated anti-rabbit and anti-mouse IgG were used as secondary antisera (1:1000; Vector Laboratories, Burlingame, CA, USA). The sections were further incubated in alkaline phosphatase-streptavidin (1:1000; Vector Laboratories), and reacted with Fast Red Substrate System (Dako).
In the experiment, all sections were thoroughly rinsed in PBS between each step and were finally counterstained with Mayer's hematoxylin and mounted in glycerol gelatin (Sigma, St. Louis, MO, USA).
Sections of human cutaneous melanoma were used as positive control tissues for survivin and p53 staining; negative controls were obtained by omission of the primary antibody or by replacing the primary antibody with an isotypematched antibody. Positive and negative controls were run simultaneously.
Micrographs were captured by a digital camera Canon PowerShot A620 (Canon Inc., Tokyo, Japan) on a microscope Zeiss Axiophot (Carl Zeiss Inc., Oberkochen, Germany), and processed by Adobe Photoshop software (version 7.0; Adobe Systems, Inc., San Jose, CA, USA).
Evaluation of immunoreactivity
Results were independently evaluated by three researchers (M.T.P., C.M. and P.D.) in a blinded fashion. Four to six ×200 fields covering almost the whole of each of the four sections per sample were examined with a 144-intersection point square reticulum (0.78 mm2) inserted in the eyepiece and scored for the percentage of immunoreactive cells.
The cutoff level for the immunohistochemical analysis was set at 10%, meaning that those samples with >10% of cells showing a moderate/strong intensity of nuclear and cytoplasmic staining were considered to be positive.
Statistical analysis
Follow-up data were analyzed in September 2007. Overall survival (OS) and disease-free survival (DFS) were calculated based on the date of initial primary chemotherapy and were analyzed using the Kaplan-Meier method. Statistical comparisons between groups were performed using two-sided log-rank tests. OS was defined as the time from study entry to the time of death from any cause. DFS was defined as the time from study entry to the time of local, regional or distant treatment failure, occurrence of controlateral breast cancer or other second primary cancer; or death without evidence of breast or a second primary cancer. Multivariate analysis was performed using the Cox regression model to assess additional prognostic values of the different variables in relation to the expression of survivin.
Data were computed by the SPSS statistical software package, version 15.0 (SPSS Inc., Chicago, IL, USA). All tests were two-tailed, and a p-value of <0.05 was considered statistically significant.
Results
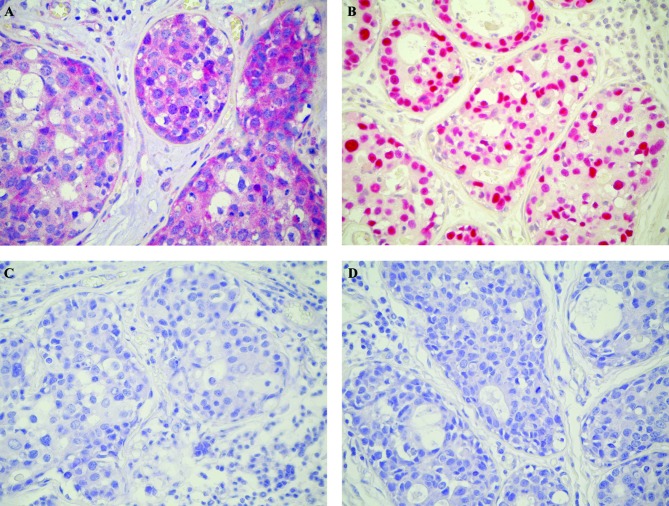
All 53 patients were able to complete the multimodality plan and therefore were evaluable for response to primary chemotherapy. During neoadjuvant therapy, no case of progressive disease was observed. The clinical response rate was 100% (95% CI, 65.2–89.5): complete clinical response (cCR) was observed in 8 patients (15%) and partial clinical response (cPR) in 45 patients (85%). According to Sataloff's classification, pathological complete response in the primary tumor (pCR) was observed in 6 patients (11%), whereas pathological gross residual tumor (pTR) was observed in 47 patients (89%). Major pathological response in breast tissue was observed in 18 patients (34%). The pathological lymph node (LN) assessment showed 12 patients with pLN0 (23%) and 41 patients with pLN+ (77%). The expression of survivin protein was positive in 21 patients (40%) and negative in 32 patients (60%); p53 immunostaining was detected in 13 cases (25%) and 40 patients (75%) did not show any staining. Eleven patients (21%) were both survivin- and p53-positive (Fig. 1).
Figure 1.
Immunohistochemical staining for survivin and p53 in T4 breast cancer. Intense survivin immunoreactivity in tumoral cells (A). Presence of strong nuclear p53 expression (B). In control sections (C and D) the immunoreactivity was completely abolished. Counterstained with hematoxylin. Original magnification ×400.
Global 5- and 10-year overall survival and disease-free survival
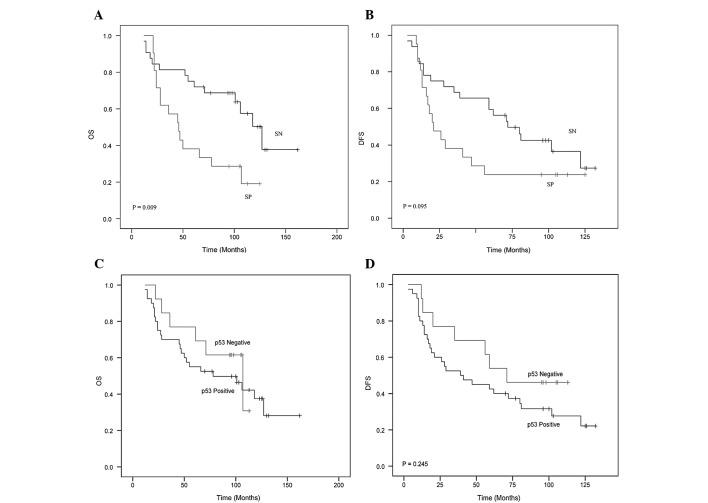
In the entire group of 53 patients treated with primary chemotherapy, the 5- and 10-year OS was 60.4 and 43.4% respectively. The 5- and 10-year OS in the survivin-negative patients was 75 and 56.3% respectively. The 5- and 10-year OS in the survivin-positive patients was 38 and 23.8%, respectively (p=0.009) (Fig. 2A).
Figure 2.
Ten-year overall survival (OS) (A and C) and disease-free survival (DFS) (B and D) for T4 breast cancer patients with survivin (A and B) and p53 expression (C and D) as determined using the Kaplan-Meier method.
The overall 5- and 10-year DFS was 45 and 32.1%, respectively. The 5- and 10-year DFS in the 32 survivin-negative patients was 59.4 and 37.5%, respectively. The 5- and 10-year DFS in the 21 survivin-positive patients was 23.8% (p=0.095) (Fig. 2B).
Among the patients with p53-positive or -negative expression, no statistically significant differences were observed in terms of the 5- and 10-year DFS and OS (Fig. 2C and D).
Effect of independent predictors of long-term overall survival in relation to the expression of survivin
The statistical analysis performed using the Cox-regression model on the patients with survivin-positivity showed a hazard ratio (HR) of 2.6 (95% CI, 1.2–5.5; p=0.012). When survivin-positivity was associated with several prognostic variables (age, ER, PR, G2/3, Ki-67, stage T4d and HER2), the HR ranged between 2.3 and 2.6. When survivin-positivity was associated with the variable p53, the HR was 3.27 (95% CI, 1.5–7.2) (Table II).
Table II.
Effect of predictors on the 10-year OS in relation to the expression of survivin in a multivariate analysis performed using the Cox-regression model.
| Predictors | HR | 95% CI | p-value | |
|---|---|---|---|---|
| Survivin-positivea | 2.615 | 1.240–5.515 | 0.012 | |
| Ageb | SP | 2.609 | 1.236–5.504 | 0.012 |
| ERb | SP | 2.343 | 1.083–5.070 | 0.031 |
| PgRb | SP | 2.265 | 1.030–4.982 | 0.042 |
| G2/3b | SP | 2.650 | 1.258–5.581 | 0.010 |
| HER2 statusb | SP | 2.612 | 1.238–5.509 | 0.012 |
| p53-positiveb | SP | 3.279 | 1.491–7.213 | 0.003 |
| T4db | SP | 2.565 | 1.204–5.464 | 0.015 |
| Triple negativeb | SP | 2.450 | 1.305–5.657 | 0.022 |
| Ki-67b | SP | 2.473 | 1.162–5.265 | 0.019 |
Survivin positivity (SP) was analyzed as an independent single variable.
The variables displayed in the first column, one at a time, were analyzed for an association with survivin positivity. HR, hazard ratio; CI, confidence interval; ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Discussion
The primary objective of our study was to investigate the correlation between the expression of survivin protein with long-term survival in a homogeneous group of stage T4 breast cancer patients treated with a multimodality treatment.
Survivin was detected in the nucleus and cytoplasm. In these cases, the nuclear reaction was prominent with a low cytoplasmic reaction. Other cases exhibited staining confined exclusively to the cytoplasm. Mitotic figures were stained, and survivin was also localized to mitotic spindles.
It is known that there are different survivin splice variants with unique subcellular localizations and functions (17). Among these, nuclear variants appears to play a key role in cell division, whereas cytoplasmic varients seem essential for the inhibition of apoptosis (18).
It is not yet possible to quantify and study the survivin proteins individually due to the lack of specific antibodies (19). Thus, our immunohistochemical localization in both the nuclei and cytoplasm, obtained by an antibody recognizing all of the survivin proteins, represented the combined expression of all variants.
In regards to potential prognostic factors, nuclear or cytoplasmic survivin has been demonstrated to be an unfavorable prognostic marker in several types of tumors, while other studies have described nuclear or cytoplasmic survivin as a favorable prognostic marker (20–24).
In our study, the statistical analysis showed that the patients whose tumors did not express survivin had a better outcome compared to survivin-positive tumors in terms of either DFS or OS. These findings are not surprising in view of other works showing that survivin expression is associated with a worse outcome in several tumor types. Regarding breast cancer, as we previously mentioned, there is still controversy on the exact value of survivin as a prognostic factor.
In this regard, Kennedy et al (21) found a negative correlation between survivin expression and survival, while the results of Tanaka et al (12) appeared consistent with our findings since survivin positivity was associated with a worse outcome in a non-homogeneous series of patients with stage I–III breast cancer. Similar results were also reported by Hinnis et al (11) who found that survivin positivity was significant associated with poor survival in a series of breast cancer patients treated with chemotherapy or hormone therapy.
In our study, which differed from others because of a longer median follow-up period (125 months), we demonstrated, in a homogeneous series of T4 breast cancer patients, that the expression of survivin is associated with a significantly shorter duration of overall survival [HR=2.6 (95% CI, 1.2–5.5)] (p=0.012) even long-term.
Moreover, additional prognostic factors were analyzed to evaluate their influence when associated, one by one, with survivin positivity. In more detail, none of the prognostic factors considered in this study except the variable p53 modified the HR. Notably, p53 (HR=3.2) seemed to negatively enhance the effect of survivin on survival. When we analyzed the outcome of patients whose tumors were both p53- and survivin-positive, a significant less favorable prognosis was observed. This finding may be explained taking into consideration the tight relationship between wild-type p53 and survivin which act in an opposite manner (25). In fact, p53 negatively regulates the expression of survivin. Conversely, the presence of mutant p53 would translate with increased expression of survivin, leading to the speculation that the combination of these two molecular factors might have a synergistic negative effect on survival.
In conclusion, our study demonstrated that survivin expression in T4 breast cancer patients is a possible independent prognostic factor which is maintained long-term. In the future, additional large prospective studies are needed to validate the expression of survivin as a potential novel biomarker for breast cancer patients.
Acknowledgments
Particular thanks are due to Mrs Maria Itala Mosso and Mr Massimo Annis for their expert technical assistance.
References
- 1.Ferlay J, Bray F, Pisani P, Parkin DM, GLOBOCAN 2002 . Cancer Incidence, Mortality and Prevalence Worldwide. IARC Press; Lyon: 2004. [Google Scholar]
- 2.Dowsett M, Dunbier AK. Emerging biomarkers and new understanding of traditional markers in personalized therapy for breast cancer. Clin Cancer Res. 2008;14:8019–8026. doi: 10.1158/1078-0432.CCR-08-0974. [DOI] [PubMed] [Google Scholar]
- 3.Altieri DC. Molecular cloning of effector cell protease receptor-1, a novel cell surface receptor for the protease factor Xa. J Biol Chem. 1994;269:3139–3142. [PubMed] [Google Scholar]
- 4.Altieri DC. Splicing of effector cell protease receptor-1 mRNA is modulated by an unusual retained intron. Biochemistry. 1994;33:13848–13855. doi: 10.1021/bi00250a039. [DOI] [PubMed] [Google Scholar]
- 5.Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosini G, Adida C, Altieri DC. A novel antiapoptosis gene, survivin expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 7.Adida C, Crotty PL, McGrath J, et al. Developmentary regulated expression of the novel cancer antiapoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Olie RA, Simões-Wüst AP, Baumann B, et al. A novel antisense oligonucleotide targeting survivin expression induces apoptosis and sensitizes lung cancer cells to chemotherapy. Cancer Res. 2000;60:2805–2809. [PubMed] [Google Scholar]
- 9.Sah NK, Khan Z, Khan GJ, Bisen PS. Structural, functional and therapeutic biology of survivin. Cancer Lett. 2006;244:164–171. doi: 10.1016/j.canlet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 10.O'Driscoll L, Linehan R, Kennedy MS, et al. Lack of prognostic significance of survivin, survivin-ΔEx3, survivin-2B, galectin-3, bag-1, bax-α and MRP-1 mRNAs in breast cancer. Cancer Lett. 2003;201:225–236. doi: 10.1016/s0304-3835(03)00518-4. [DOI] [PubMed] [Google Scholar]
- 11.Hinnis AR, Luckett JCA, Walker RA. Survivin is an independent predictor of short-term survival in poor prognostic breast cancer patients. Br J Cancer. 2007;96:639–645. doi: 10.1038/sj.bjc.6603616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Iwamoto S, Gon G, et al. Expression of survivin and its relationship to loss of apoptosis in breast carcinomas. Clin Cancer Res. 2000;6:127–134. [PubMed] [Google Scholar]
- 13.Bertheau P, Espié M, Turpin E, et al. TP53 status and response to chemotherapy in breast cancer. Pathobiology. 2008;75:132–139. doi: 10.1159/000123851. [DOI] [PubMed] [Google Scholar]
- 14.Brennan DJ, Rexhepaj E, O'Brien SL, et al. Altered cytoplasmic-to-nuclear ratio of survivin is a prognostic indicator in breast cancer. Clin Cancer Res. 2008;14:2681–2689. doi: 10.1158/1078-0432.CCR-07-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayward JL, Carbone PP, Heusen JC, et al. Assessment of response to therapy in advanced breast cancer. Br J Cancer. 1977;35:292–298. doi: 10.1038/bjc.1977.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sataloff DM, Mason BA, Prestipino AJ, et al. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180:297–306. [PubMed] [Google Scholar]
- 17.Li F, Ling X. Survivin study: an update of ‘what is the next wave’? J Cell Physiol. 2006;208:476–486. doi: 10.1002/jcp.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohi T, Beltrami E, Wall NR, Plescia J, Altieri DC. Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest. 2004;114:1117–1127. doi: 10.1172/JCI22222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XN, Shu Q, Su JM, et al. Differential expression of survivin splice isoforms in medulloblastomas. Neuropathol Appl Neurobiol. 2007;33:67–76. doi: 10.1111/j.1365-2990.2006.00782.x. [DOI] [PubMed] [Google Scholar]
- 20.Altieri DC, Marchisio PC. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327–1333. [PubMed] [Google Scholar]
- 21.Kennedy SM, O'Driscoll L, Purcell R, et al. Prognostic importance of survivin in breast cancer. Br J Cancer. 2003;88:1077–1083. doi: 10.1038/sj.bjc.6600776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ponnelle T, Chapusot C, Martin L, et al. Cellular localisation of survivin: impact on the prognosis in colorectal cancer. J Cancer Res Clin Oncol. 2005;131:504–510. doi: 10.1007/s00432-005-0682-z. [DOI] [PubMed] [Google Scholar]
- 23.Xie D, Zeng YX, Wang HJ, et al. Expression of cytoplasmic and nuclear survivin in primary and secondary human glioblastoma. Br J Cancer. 2006;94:108–114. doi: 10.1038/sj.bjc.6602904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piras F, Murtas D, Minerba L, et al. Nuclear survivin is associated with disease recurrence and poor survival in patients with cutaneous malignant melanoma. Histopathology. 2007;50:835–842. doi: 10.1111/j.1365-2559.2007.02695.x. [DOI] [PubMed] [Google Scholar]
- 25.Mirza A, McGuirk M, Hockenberry TN, et al. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]