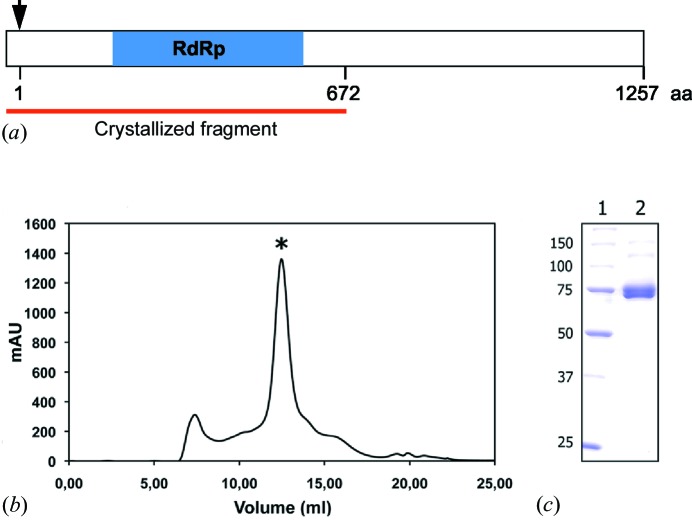
Figure 1.
Production and purification of the hTaV RdRP. (a) Schematic representation of the recombinant hTaV ORF1 construct. The black arrow indicates the first residue of the native viral protein and the blue box shows the region containing the conserved motifs in all RdRps (Gorbalenya et al., 2002 ▶). The red line indicates the crystallized fragment (672 residues of the viral polypeptide plus the fusion tag). (b) Size-exclusion chromatogram (Superdex 200 HR 10/300) of the purified polypeptide. According to the pre-calibration of the column, the protein elutes in a volume corresponding to a dimer of the RdRp domain (*). A small amount of aggregates were also visible. mAU on the y axis indicates the relative absorbance units at 280 nm. (c) SDS–PAGE of the purified protein after Superdex 200 chromatography. Lane 1 shows the molecular-weight marker (kDa) and lane 2 contains the sample peak, labelled with ‘*’ in the chromatogram.