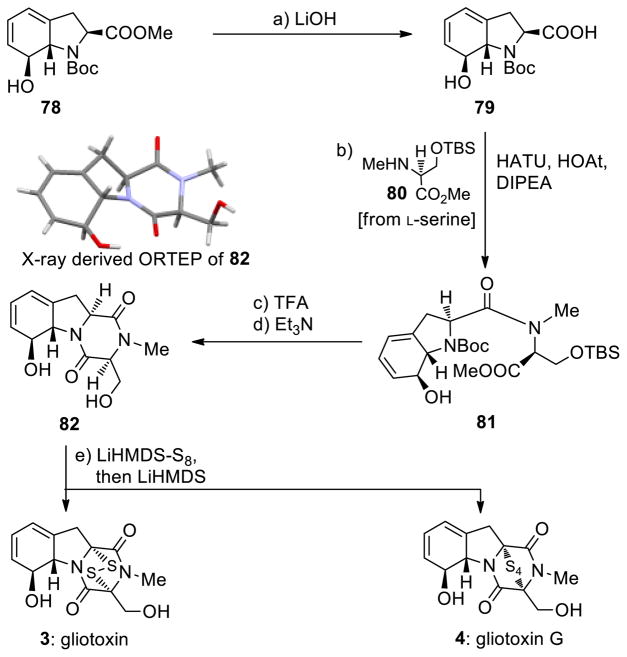
Scheme 10.
Completion of the Enantioselective Total Syntheses of Gliotoxin (3) and Gliotoxin G (4)a
aReagents and conditions. a) aq. LiOH (1.0 M)/THF (6:1), 0→25 °C, 5 h, 99%; b) 79 (1.0 equiv), 80 (2.0 equiv), HOAt (1.1 equiv), HATU (1.1 equiv), DIPEA (3.0 equiv), CH2Cl2, 0→25 °C, 15 h, 88%; c) TFA/CH2Cl2 (1:1), 0→25 °C, 3 h; d) Et3N (5.0 equiv), CH2Cl2, 0→25 °C, 15 h, 63% for the two steps; e) LiHMDS (1.0 M in THF, 4.0 equiv), S8 (8.0 equiv), THF, 25 °C, 5 min; then 82 (0.06 M in THF, 1.0 equiv) 5 min; then LiHMDS (1.0 M in THF, 4.0 equiv), 25 °C, 1.5 h, 3: 23%, 4: 33%, plus 6% recovered starting material 82.