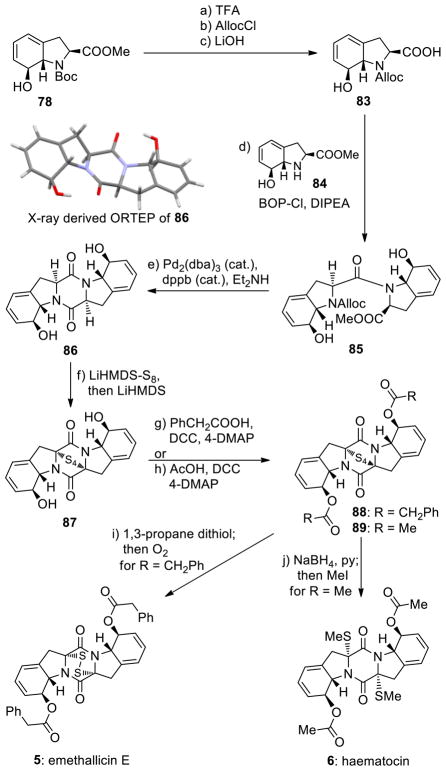
Scheme 11.
Completion of the Enantioselective Total Syntheses of Emethallicin E (5) and Haematocin (6)a
aReagents and conditions. a) TFA/CH2Cl2 (1:2.5), 25 °C, 4 h, 95%; b) AllocCl (1.7 equiv), NaHCO3 (10.0 equiv), dioxane/H2O (1:1), 0→25 °C, 3 h, 88%; c) LiOH aq. (1.0 M)/THF (1:1), 0→25 °C, 5 h; d) 83, 84 (1.0 equiv each), BOP-Cl (1.1 equiv), DIPEA (3.0 equiv), CH2Cl2, 0→25 °C, 15 h, 83% for the two steps; e) Pd2(dba)3 (0.02 equiv), dbbp (0.05 equiv), THF/Et2NH (2:1), 25 °C, 2 h, 84%; f) LiHMDS (1.0 M in THF, 20 equiv), S8 (37 equiv), THF, 25 °C, 5 min; then 86 (0.06 M in THF/Et2O (9:1), 1.0 equiv), 5 min; then LiHMDS (1.0 M in THF, 20 equiv), 25 °C, 5 h, 46%, plus 43% recovered starting material 86; g) PhCH2COOH (30 equiv), DCC (30 equiv), 4-DMAP (3.0 equiv), 0→25 °C, 15 h, 71%, plus 26% recovered starting material 87; h) AcOH (30 equiv), DCC (30 equiv), 4-DMAP (3.0 equiv), 0→25 °C, 15 h, 71%, plus 24% recovered starting material 87; i) 1,3-propane dithiol (90 equiv), Et3N (0.32 equiv), MeCN/CH2Cl2 (25:1), 25 °C; then concentrate; then O2, MeOH, 2 h, 25 °C, 54% overall; j) NaBH4 (80 equiv), MeOH/py (1:1), 0 °C; then MeI (485 equiv) 0→25 °C, 4 h; 97% overall.