Table 1.
Preparation of Epidithiodiketopiperazinesa
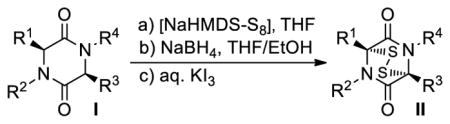
| |||
|---|---|---|---|
| Entry | Substrate | Productb | Overall Yield [%]c |
| 1 |
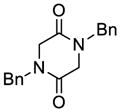 29 |
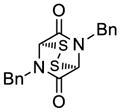 30 |
40 |
| 2 |
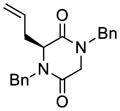 31 |
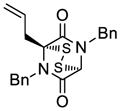 32 |
63 |
| 3 |
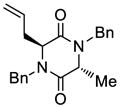 33 |
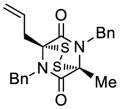 34 |
70 |
| 4 |
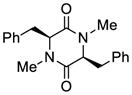 24 |
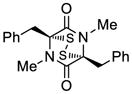 27 |
69 |
| 5 |
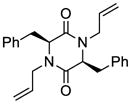 35 |
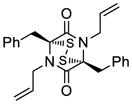 36 |
65 |
| 6 |
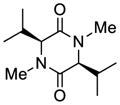 37 |
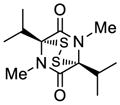 38 |
45 |
| 7 |
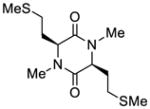 39 |
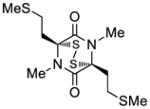 40 |
43 |
| 8 |
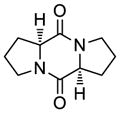 41 |
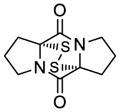 42 |
65 |
| 9 |
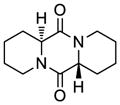 43 |
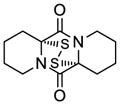 44 |
68 |
| 10 |
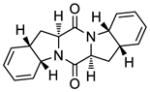 45 |
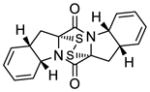 46 |
55d |
| 11 |
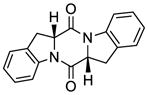 47 |
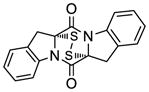 48 |
68 |
| 12 |
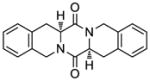 49 |
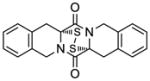 50 |
70 |
Reactions were performed on 100 mg scale at 25 °C.
Racemic mixture unless otherwise stated.
Yield of isolated products after chromatography.
ca. 1.4:1 dr.
