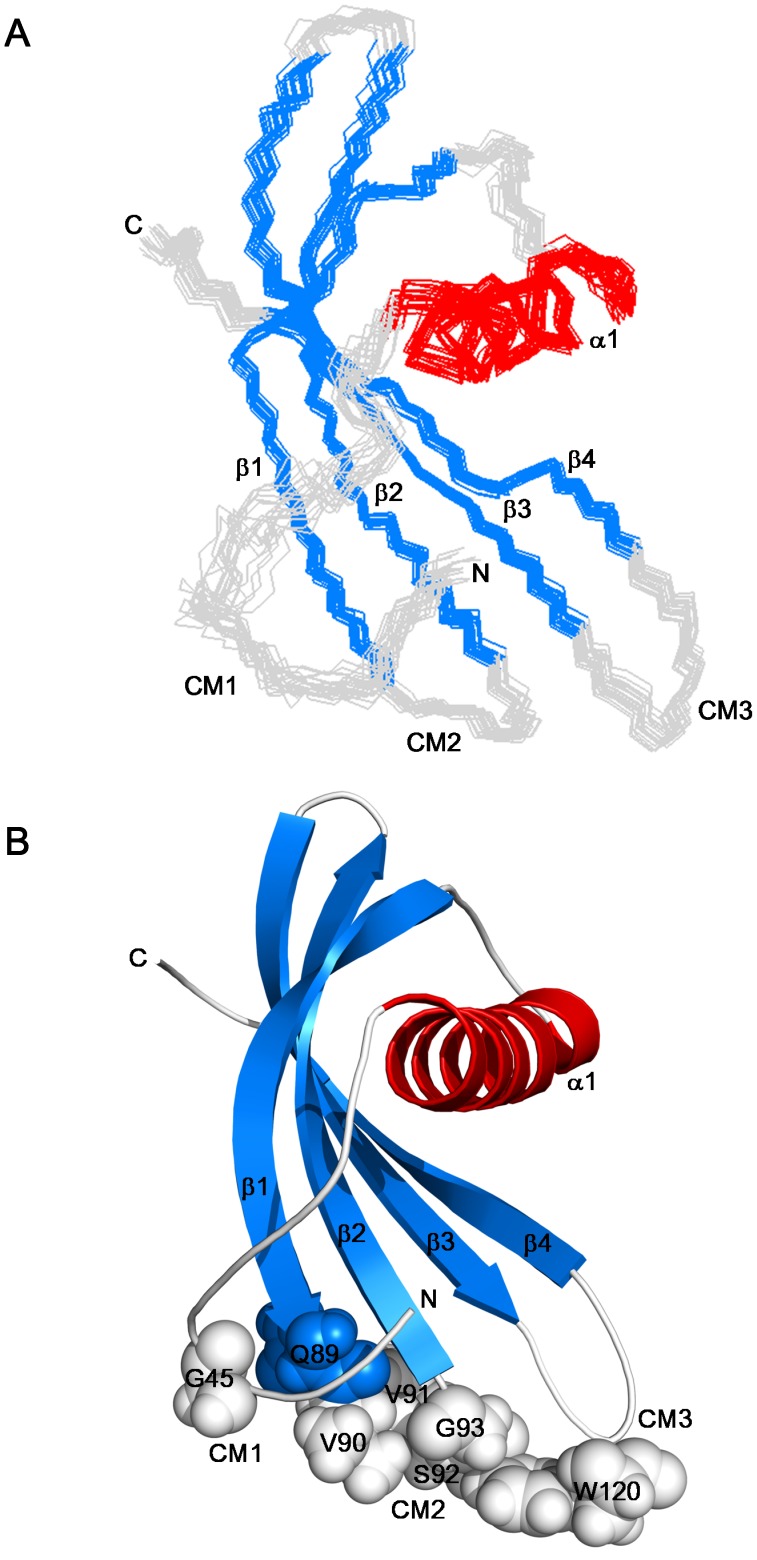
Figure 2. Solution structure of AcCYS.
(A) The ensemble of 20 best structures of the inhibitory domain of AcCYS (res 41 to 135) is superimposed. The α-helical, β-sheet, and loop region is color coded in red, green, and grey respectively. (B) Ribbon representation of the inhibitory domain of AcCYS structure shows a αβ roll structure made up of one α-helix and four anti-parallel β-strands, β1–β4. Three regions which contain the highly conserved motifs are labeled as CM1–3. Side chains of these highly conserved residues including, G45, Q89VVSG, and W120 are shown in spheres. These diagrams were generated using the structural visualization program PYMOL.