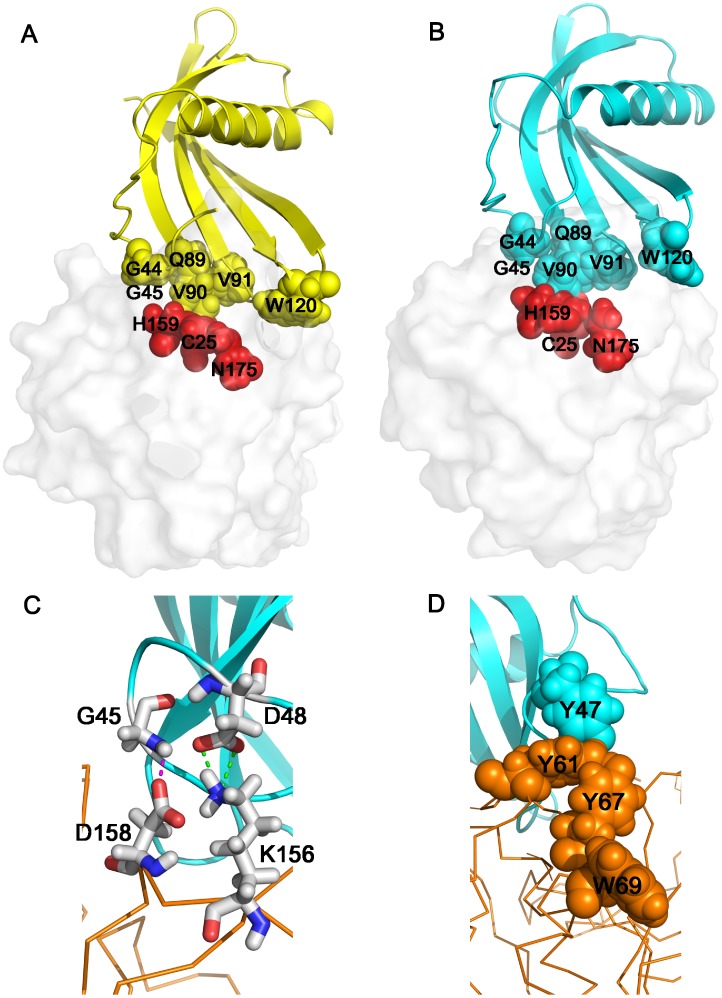
Figure 8. Structural model of AcCYS/papain complex.
(A) The complex structure of AcCYS/papain was simulated by ZDOCK. AcCYS is shown in yellow ribbons. The binding site of AcCYS is shown in yellow spheres. The surface of papain is shown in light grey. The active site of papain including C25, H159, and N175 residues are shown in red spheres. (B) Complex structure of AcCYS/papain determined by restrained molecular dynamics simulations. AcCYS is shown in light blue ribbons. The G44–G45 and Q89–V91 of AcCYS shown in spheres completely block the active site of papain. The first turn of α-helix, residue 56–58 collapses. (C) AcCYS/papain complex stabilized by intermolecular forces. The backbone of papain is shown in yellow line. An intermolecular H-bond between G45 amide proton of AcCYS and the side chain carboxyl of D158 of papain and an intermolecular salt bridge between the carboxyl of D48 of AcCYS and K156 of papain are formed in the simulated complex structure (shown in sticks). (D) A new hydrophobic cluster between AcCYS and papain. The side chain of Y47 of AcCYS (shown in light blue spheres) as well as Y61, Y66, and W69 residues of papain (shown in yellow spheres) form an intermolecular aromatic cluster.