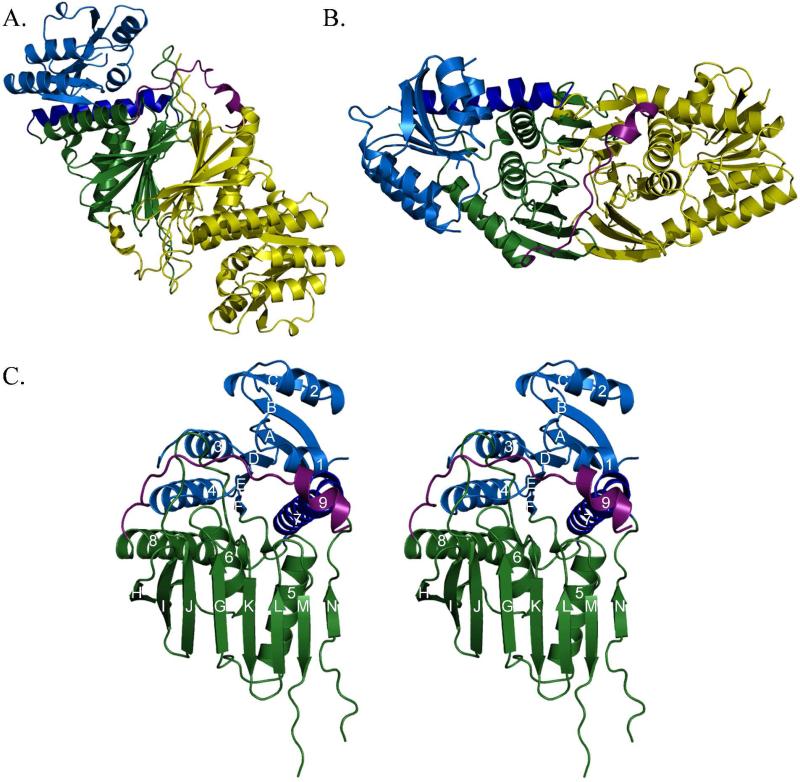
Figure 2. Overall architecture of Irp3.
(A and B) Dimeric structure of Irp3. The A monomer is shown in yellow. The B monomer is shown in blue (N-terminal domain) and green (C-terminal domain), with the C-terminal tail shown in magenta. Parts (A) and (B) are related by a 90° rotation about the x-axis. The dimerization interface is evident where the open sides of the β-sheet in the C-terminal domains pack together. The magenta helix of the C-terminal tail packs against the opposing monomer, also contributing to the dimerization interface. Monomers A and B of the native structure are depicted. (C) Stereoview of monomer. The N-terminal, NADP(H) binding domain (residues 1-125) is blue, with the helix donated from the C-terminus shown in dark blue (residues 285-310). The C-terminal substrate binding domain is shown in green (residues 126-284 and 311 – 338). The C-terminal tail is shown in magenta. Strands are denoted by letters A – N, starting from the N-terminus. Helices are counted from the N-terminus, 1-8. Monomer A of the native structure is depicted.