Abstract
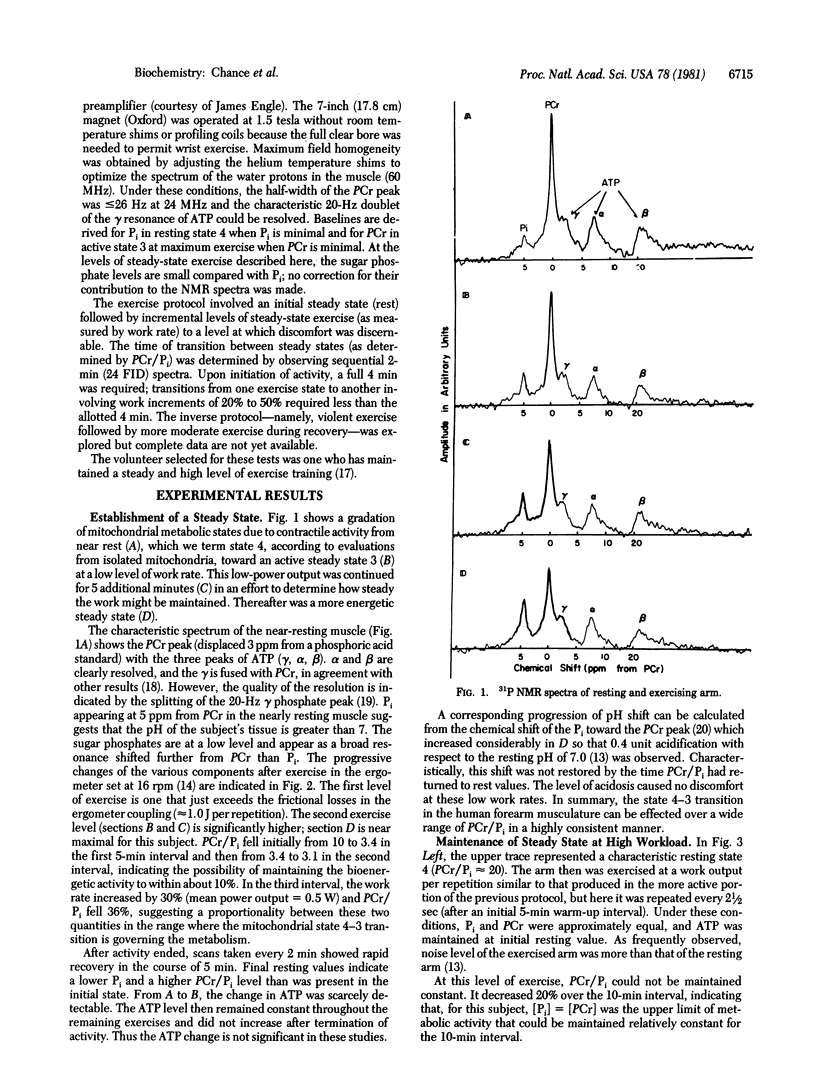
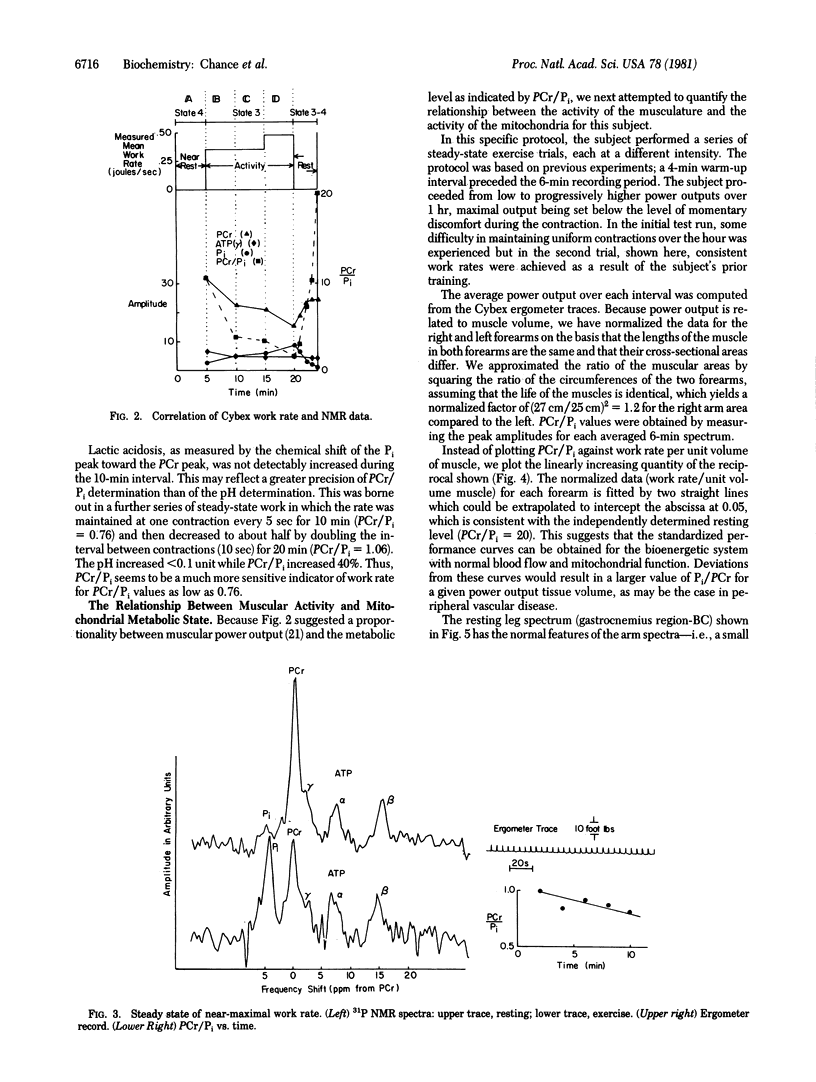
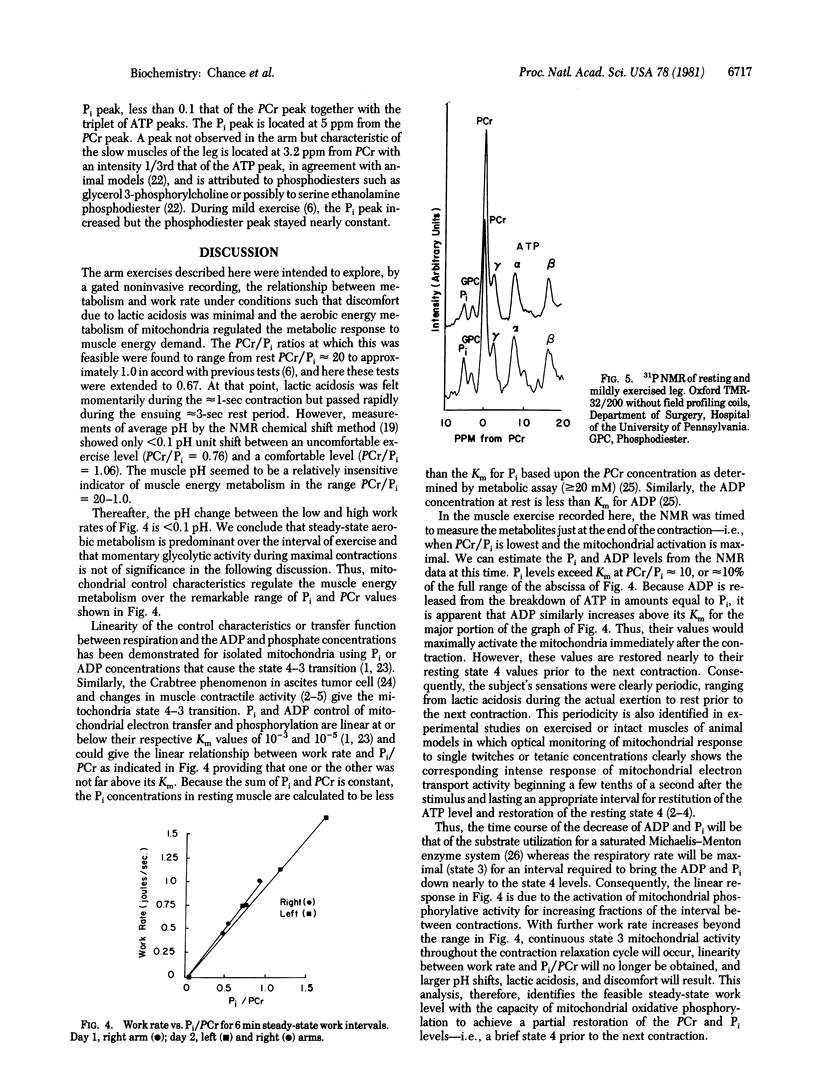
31P NMR is used to determine the relationship between work output in the exercising human forearm and the steady-state capability of oxidative phosphorylation as measured by the phosphocreatine/inorganic phosphate ratio (PCr/Pi). Exercise intensities (one contraction per 5 sec) permitting comfortable continuation of activity for greater than 1 hr produced PCr/Pi of about 1 for a subject of moderate training. Linear relationships between work rate per unit volume of muscle and the 5-min mean PCr/Pi were found for the subject's left and right arms. The protocol affords sensitive criteria of muscle performance in normal subjects and of biochemical or vascular disease in abnormal subjects. The Pi, PCr, and ATP levels found by 31P NMR represent the initial values in the cycle of contraction and relaxation which permit restitution of resting state 4 prior to the next contraction and the continuation of steady-state work performance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHANCE B., WEBER A. THE STEADY STATE OF CYTOCHROME B DURING REST AND AFTER CONTRACTION IN FROG SARTORIUS. J Physiol. 1963 Nov;169:263–277. doi: 10.1113/jphysiol.1963.sp007255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- COHN M., HUGHES T. R., Jr Phosphorus magnetic resonance spectra of adenosine di- and triphosphate. I. Effect of pH. J Biol Chem. 1960 Nov;235:3250–3253. [PubMed] [Google Scholar]
- Chalovich J. M., Burt C. T., Danon M. J., Glonek T., Bárány M. Phosphodiesters in muscular dystrophies. Ann N Y Acad Sci. 1979;317:649–669. doi: 10.1111/j.1749-6632.1979.tb56585.x. [DOI] [PubMed] [Google Scholar]
- Chance B., Eleff S., Leigh J. S., Jr Noninvasive, nondestructive approaches to cell bioenergetics. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7430–7434. doi: 10.1073/pnas.77.12.7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadian D. G., Radda G. K., Brown T. R., Chance E. M., Dawson M. J., Wilkie D. R. The activity of creatine kinase in frog skeletal muscle studied by saturation-transfer nuclear magnetic resonance. Biochem J. 1981 Jan 15;194(1):215–228. doi: 10.1042/bj1940215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick P. B., Radda G. K., Seeley P. J. Studies of acidosis in the ischaemic heart by phosphorus nuclear magnetic resonance. Biochem J. 1979 Dec 15;184(3):547–554. doi: 10.1042/bj1840547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollnick P. D., Piehl K., Saubert C. W., 4th, Armstrong R. B., Saltin B. Diet, exercise, and glycogen changes in human muscle fibers. J Appl Physiol. 1972 Oct;33(4):421–425. doi: 10.1152/jappl.1972.33.4.421. [DOI] [PubMed] [Google Scholar]
- HESS B., CHANCE B. Metabolic control mechanisms. VI. Chemical events after glucose addition to ascites tumor cells. J Biol Chem. 1961 Jan;236:239–246. [PubMed] [Google Scholar]
- Karlsson J. Lactate and phosphagen concentrations in working muscle of man with special reference to oxygen deficit at the onset of work. Acta Physiol Scand Suppl. 1971;358:1–72. [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Moffroid M., Whipple R., Hofkosh J., Lowman E., Thistle H. A study of isokinetic exercise. Phys Ther. 1969 Jul;49(7):735–747. doi: 10.1093/ptj/49.7.735. [DOI] [PubMed] [Google Scholar]
- Moon R. B., Richards J. H. Determination of intracellular pH by 31P magnetic resonance. J Biol Chem. 1973 Oct 25;248(20):7276–7278. [PubMed] [Google Scholar]
- Pernow B., Saltin B., Wahren J., Cronestrand R., Ekestroöm S. Leg blood flow and muscle metabolism in occlusive arterial disease of the leg before and after reconstructive surgery. Clin Sci Mol Med. 1975 Sep;49(3):265–275. doi: 10.1042/cs0490265. [DOI] [PubMed] [Google Scholar]
- Ross B. D., Radda G. K., Gadian D. G., Rocker G., Esiri M., Falconer-Smith J. Examination of a case of suspected McArdle's syndrome by 31P nuclear magnetic resonance. N Engl J Med. 1981 May 28;304(22):1338–1342. doi: 10.1056/NEJM198105283042206. [DOI] [PubMed] [Google Scholar]
- SERAYDARIAN K., MOMMAERTS W. F., WALLNER A., GUILLORY R. J. An estimation of the true inorganic phosphate content of frog sartorius muscle. J Biol Chem. 1961 Jul;236:2071–2075. [PubMed] [Google Scholar]
- Sahlin K. Intracellular pH and energy metabolism in skeletal muscle of man. With special reference to exercise. Acta Physiol Scand Suppl. 1978;455:1–56. [PubMed] [Google Scholar]


