Abstract
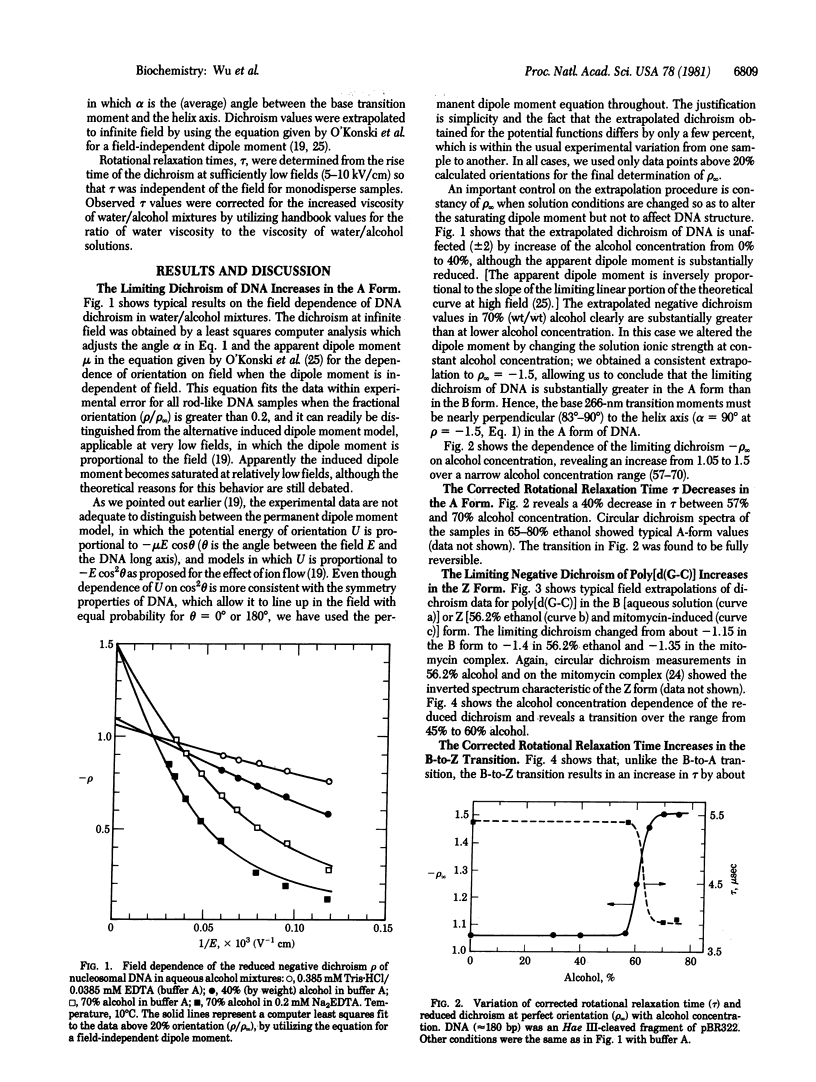
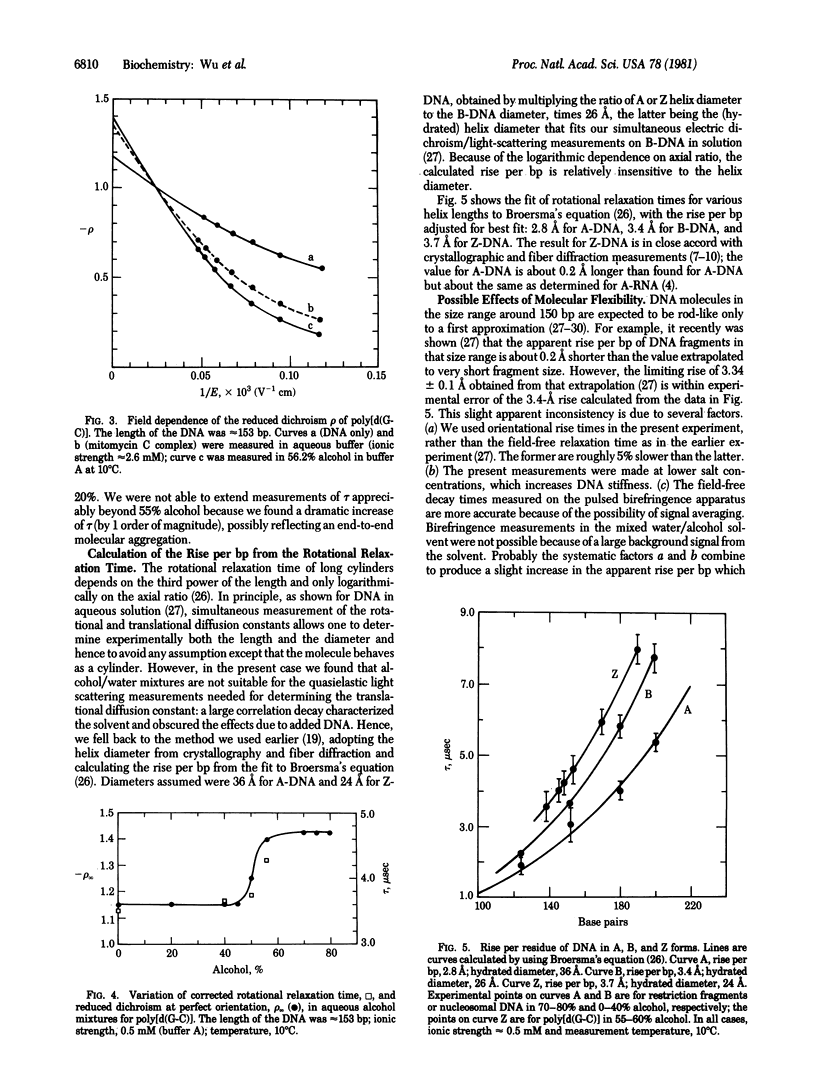
We report transient electric dichroism studies of short double-helical DNA and poly[d(G-C)] fragments in alcohol/water mixtures. The limiting reduced dichroism and the rotational correlation time changed abruptly in the alcohol concentration range expected for the DNA B-to-A and poly[d(G-C)] B-to-Z transitions. The Z form of poly[d(G-C)] was also induced by mitomycin C crosslinking in aqueous solution. The rotational correlation times observed for A- and Z-DNA were approximately consistent with dimensions determined by crystallographic and fiber diffraction analysis: the estimated rise per base pair was 2.8 A for A-DNA and 3.7 A for Z-DNA in solution. In addition, the observed limiting reduced dichroism values for A- and Z-DNA were close to the theoretical limit of -1.5, requiring a structure in which the base transition moments are effectively perpendicular to the double-helix axis. This is the result expected for any DNA double helix having dyad symmetry in which the base pairs are flat and the base transition moments lie predominantly in the short axis of the base pair and therefore close to a helix dyad axis. Only B-DNA deviates from this rule, strongly reinforcing our earlier conclusion that the base pairs in B-DNA are not flat but are propeller twisted, either statically or as a dynamic average.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Chandrasekaran R., Birdsall D. L., Leslie A. G., Ratliff R. L. Left-handed DNA helices. Nature. 1980 Feb 21;283(5749):743–745. doi: 10.1038/283743a0. [DOI] [PubMed] [Google Scholar]
- Arnott S., Hukins D. W. Optimised parameters for A-DNA and B-DNA. Biochem Biophys Res Commun. 1972 Jun 28;47(6):1504–1509. doi: 10.1016/0006-291x(72)90243-4. [DOI] [PubMed] [Google Scholar]
- Arnott S., Wilkins M. H., Fuller W., Langridge R. Molecular and crystal structures of double-helical RNA. 3. An 11-fold molecular model and comparison of the agreement between the observed and calculated three-dimensional diffraction data for 10- and 11-fold models. J Mol Biol. 1967 Aug 14;27(3):535–548. doi: 10.1016/0022-2836(67)90057-5. [DOI] [PubMed] [Google Scholar]
- Bram S., Baudy P. X-ray diffraction studies of DNA at reduced water contents. Nature. 1974 Aug 2;250(465):414–416. doi: 10.1038/250414a0. [DOI] [PubMed] [Google Scholar]
- Crawford J. L., Kolpak F. J., Wang A. H., Quigley G. J., van Boom J. H., van der Marel G., Rich A. The tetramer d(CpGpCpG) crystallizes as a left-handed double helix. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4016–4020. doi: 10.1073/pnas.77.7.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattagupta N., Crothers D. M. Solution structural studies of the Ag(I)-DNA complex. Nucleic Acids Res. 1981 Jun 25;9(12):2971–2985. doi: 10.1093/nar/9.12.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Hillen W., Wells R. D. A 300- and 600-MHz proton nuclear magnetic resonance investigation of a 12 base pair deoxyribonucleic acid restriction fragment: relaxation behavior of the low-field resonances in water. Biochemistry. 1981 Jun 23;20(13):3756–3764. doi: 10.1021/bi00516a014. [DOI] [PubMed] [Google Scholar]
- FULLER W., WILKINS M. H., WILSON H. R., HAMILTON L. D. THE MOLECULAR CONFIGURATION OF DEOXYRIBONUCLEIC ACID. IV. X-RAY DIFFRACTION STUDY OF THE A FORM. J Mol Biol. 1965 May;12:60–76. doi: 10.1016/s0022-2836(65)80282-0. [DOI] [PubMed] [Google Scholar]
- Hagerman P. J. Investigation of the flexibility of DNA using transient electric birefringence. Biopolymers. 1981 Jul;20(7):1503–1535. doi: 10.1002/bip.1981.360200710. [DOI] [PubMed] [Google Scholar]
- Herbeck R., Yu T. J., Peticolas W. L. Effect of cross-linking on the secondary structure of DNA I. Cross-linking by photodimerization. Biochemistry. 1976 Jun 15;15(12):2656–2660. doi: 10.1021/bi00657a027. [DOI] [PubMed] [Google Scholar]
- Hogan M., Dattagupta N., Crothers D. M. Transient electric dichroism of rod-like DNA molecules. Proc Natl Acad Sci U S A. 1978 Jan;75(1):195–199. doi: 10.1073/pnas.75.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Minyat E. E., Frank-Kamenetskii M. D., Schyolkina A. K. The B to A transition of DNA in solution. J Mol Biol. 1974 Aug 25;87(4):817–833. doi: 10.1016/0022-2836(74)90086-2. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Klevan L., Crothers D. M. Isolation and characterization of a spacerless dinucleosome from H1-deleted chromatin. Nucleic Acids Res. 1977 Dec;4(12):4077–4089. doi: 10.1093/nar/4.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M. How many base-pairs per turn does DNA have in solution and in chromatin? Some theoretical calculations. Proc Natl Acad Sci U S A. 1978 Feb;75(2):640–644. doi: 10.1073/pnas.75.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Canuel L. L., Pohl F. M. "Alternating B-DNA" conformation for the oligo(dG-dC) duplex in high-salt solution. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2508–2511. doi: 10.1073/pnas.76.6.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Helical periodicity of DNA determined by enzyme digestion. Nature. 1980 Aug 7;286(5773):573–578. doi: 10.1038/286573a0. [DOI] [PubMed] [Google Scholar]
- WATSON J. D., CRICK F. H. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Helical repeat of DNA in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R., Drew H., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R. E. Crystal structure analysis of a complete turn of B-DNA. Nature. 1980 Oct 23;287(5784):755–758. doi: 10.1038/287755a0. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. A direct demonstration that the ethanol-induced transition of DNA is between the A and B forms: an X-ray diffraction study. J Mol Biol. 1979 Dec 25;135(4):1023–1027. doi: 10.1016/0022-2836(79)90526-6. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Pheiffer B. H. Helical parameters of DNA do not change when DNA fibers are wetted: X-ray diffraction study. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2703–2707. doi: 10.1073/pnas.76.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]


