Fig. 2.
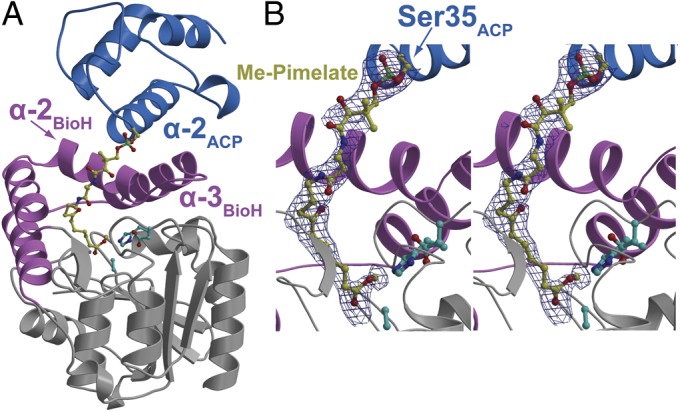
Overall structure of Me-pimeloyl-ACP in complex with BioH S82A. (A) The α/β core domain of BioH is colored gray, with the capping helices colored pink. Helices 2 and 3 of the capping domain are labeled. The BioH catalytic triad residues are shown in stick-ball representation with carbon atoms in cyan. The ACP molecule is colored blue with the helix 2 labeled. The phosphopantetheine-linked pimeloyl methyl ester is shown in stick-ball representation with carbon atoms colored yellow. (B) Zoomed-in stereoview of the phosphopantetheinylated pimeloyl methyl ester. Superimposed is a difference Fourier electron density map (contoured at 2.3σ over background in blue and 8.0σ over background in red) calculated with coefficients |Fobs| − |Fcalc| and phases from the final refined model with the coordinates of the ligand deleted before one round of refinement.
