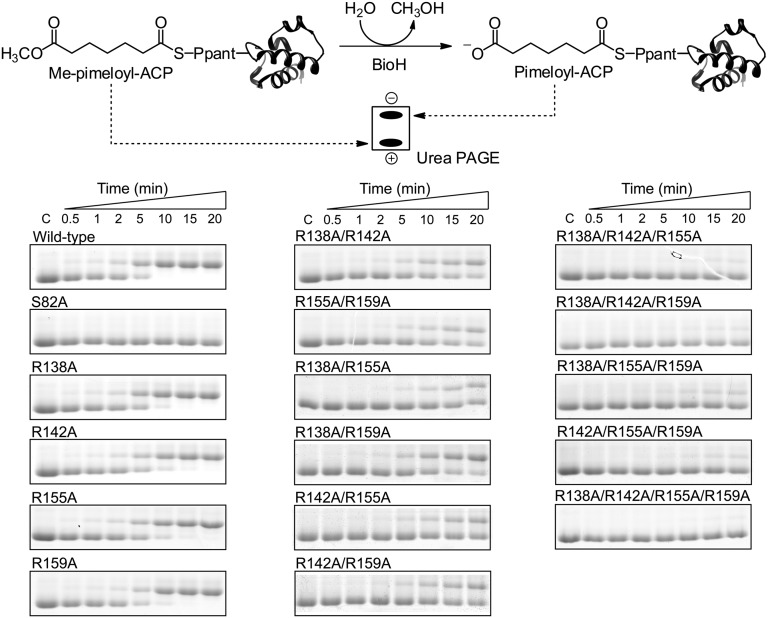
Fig. 5.
Substitution of alanine for the BioH arginine residues at positions 138, 142, 155, and 156 reduced in vitro cleavage of pimeloyl-ACP methyl ester. The product is the slower-migrating pimeloyl-ACP, which can be resolved in a destabilizing urea-PAGE system (SI Materials and Methods). Activity was assayed in vitro with 200 μM Me-pimeloyl-ACP and 5 nM enzyme for 20 min. Wild-type enzyme and single Ala substitution mutant proteins demonstrated similar catalytic rates, whereas the S82A protein was completely inactive. Double mutants showed significant reductions in activity, whereas the activities of the triple and quadruple mutants were nearly abolished. Ppant, 4′-phosphopantetheine.