Abstract
Studies with a number of viral systems have shown, on the basis of the ability of a host to prime naïve T cells, that viral antigens persist in the infected host well beyond complete clearance of the infection and even when viral antigen is undetectable by the most sensitive methods. This has led to a reasonable assumption that the antigen persists through persistence of antigen-encoding genetic information (DNA or RNA) that resides in the host at a subdetectable level. Here, we demonstrate that epitopes, or epitope precursors, of a model antigen (ovalbumin) persist in a host for prolonged periods (weeks), well beyond the time at which the intact antigen has disappeared, and in the complete absence of genetic information encoding it. Dendritic cells are shown to be the site of this epitope sequestration in vivo, as well as in cultures in vitro. For sequestration to occur, the uptaken antigen must be significantly large, that is, the epitope and its 18-mer precursor are not sequestered. Dendritic cells are shown to create an hsp90-dependent intracellular pool of epitopes or epitope precursors that continues to release epitopes for presentation on the major histocompatibility complex I molecules for prolonged periods. Demonstration of such long-term sequestration of antigenic epitopes inside dendritic cells presents new opportunities for stimulation of immune response against cancers and viruses.
Keywords: chaperone, cross-presentation, heat shock protein
A number of studies with viral systems have shown that viral antigens persist in some form for a very long time and certainly well beyond the point at which the viral infection is cleared. This is true for chronically infecting viruses, such as the respiratory syncytial (1, 2) and Sendai viruses (3), as well as lytic influenza viruses (4–8). The evidence that antigens persist comes from immunologic rather than biochemical studies: typically, it has been shown by the ability of the host, which has cleared the infection and in which no antigen is detectable, to readily support stimulation of adoptively transferred naïve T cells for weeks after the infection has been cleared. An understanding of the mechanisms of antigen persistence can shed light on several significant immunologic processes: persistence of high levels of viral antigen has been implicated in compromised immune responses to chronic infections. Additionally, antigen persistence has been linked with the nature of T-cell memory elicited in response to infection (4–9).
In explaining the mechanisms of antigen persistence, it has been reasonably argued that long after the virus is cleared, the genetic information encoding it is somehow preserved in the host at undetectable levels and its continued expression at a low level is responsible for antigen persistence. Indeed, viral transcripts have been detected long after the virus has been cleared; nonetheless, the antigen appears to persist immunologically significantly even longer after such transcripts cease to be detectable (8). Although the idea that antigen persistence is accounted for by undetectable levels of viral DNA or RNA is plausible, it is intellectually unsatisfactory.
To address this question, antigen persistence has been tested here such that naïve animals are exposed to the antigen (free or cell-associated) in the complete absence of any genetic information. Antigen persistence of the type seen in viral infections can be fully reproduced in this system; further, these studies demonstrate that antigen persists not in an intact form but as epitopes or their longer precursors that are sequestered in a depot from which they are released over a prolonged period for presentation by major histocompatibility complex (MHC) I molecules.
Results
Dendritic Cell-Dependent Epitope Persistence in Vivo.
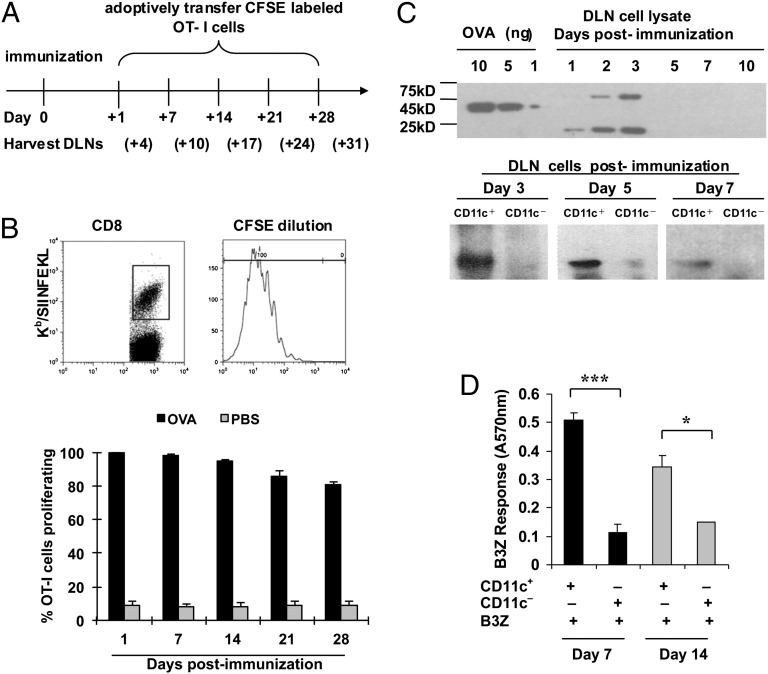
C57BL/6 mice were immunized intradermally with 200 μg soluble ovalbumin (OVA) free of detectable endotoxin (<1 EU/mg) (n = 15) or phosphate-buffered saline (PBS) (n = 15). Mice immunized with either agent were divided into five groups of three each; mice in each group were adoptive recipients of 250,000 carboxyfluorescein succinimidyl ester (CFSE)-labeled OT-I cells on days 1, 7, 14, 21, or 28 (Fig. 1A). Three days after each transfer, draining lymph nodes (DLNs) were harvested and analyzed for CFSE dilution as an indicator of proliferation of OT-I cells (Fig. 1B, Upper). In mice that were recipients of OT-I cells 1 d after immunization with soluble OVA, potent proliferation of OT-I cells was seen, whereas mice immunized with PBS showed insignificant proliferation. Surprisingly, this pattern was observed in mice that had received OT-I cells on days 7, 14, 21, or 28, after immunization on day 0. The OT-I cell proliferation diminished only very slightly (from 100 to >80%) during these 28 d (Fig. 1B, Lower).
Fig. 1.
OVA persists in vivo for several weeks after immunization. (A) Experimental design for B (Upper). Gating strategy used for CFSE-diluting CD8-positive, Kb/SIINFEKL pentamer-positive cells in drainage lymph nodes (DLNs) of immunized mice in the results shown in the lower panel. (B, Lower) Proliferation of OT-I cells measured by CFSE dilution (shown) in the DLNs of C57BL/6 mice immunized with soluble 200 μg OVA protein (black bars) or phosphate-buffered saline (gray bars). (C) OVA protein or its fragments were detected in the DLN cells (Upper) by sodium dodecyl sulfate polyacrylamide gel electrophoresis followed by immunoblotting with a polyclonal antibody at indicated time points after OVA immunization. Titrated quantities of soluble OVA (10, 5, or 1 ng) were used as quantitative standards in the left lanes. A similar analysis was conducted on CD11c-positive and CD11c-negative cells (72,000 cells each) derived from the DLNs of immunized mice 3, 5, and 7 d after immunization. (D) Stimulation of B3Z cells by CD11c-positive cells purified from DLNs of C57BL/6 mice immunized with soluble 200 μg OVA protein 7 d (black bars) or 14 d (gray bars) after immunization. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate mean ± SD. Experiments were conducted three to five times each. Three to five mice per group were used.
The persistent ability of the immunized mice to prime naïve T cells showed that OVA exists in mice in some form. Because OVA protein was used as the immunogen, persistence of residual genetic material is ruled out. DLNs of mice immunized with soluble OVA were isolated at various time points after immunization, solubilized, and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted using a polyclonal antibody to OVA that recognizes several OVA fragments. OVA or its fragments were detected in the LNs for up to 3 d but not on day 5 or later (Fig. 1C, Upper). Interestingly, an OVA-positive band, larger than the original OVA, appeared in the DLN on days 2 and 3, presumably as a result of polyubiquitination. The smaller OVA-positive band (<25 kDa) is clearly a result of degradation of ubiquitinated OVA. To define the cell populations within the DLN that may harbor the OVA fragments, the DLNs were fractionated into CD11c-positive and CD11c-negative populations; sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotting (Fig. 1C, Lower) of total cell lysates of equal numbers (∼70,000 cells each) of each cell type showed that a prominent OVA fragment is detected in CD11c-positive but not CD11c-negative cells day 3 after immunization; this band is significantly reduced in intensity by day 5 and is barely detectable day 7 after immunization. Consistent with this result, the persistence of antigen was highly dependent on CD11c-positive cells, because their depletion from the DLNs of immunized mice on day 7 or 14 led to a near-complete loss of antigen presentation to B3Z cells (Fig. 1D).
An attempt was made to determine if the antigen persists in the DCs at the site of immunization. C57BL/6 mice were immunized with OVA, as in Fig. 1, and the site of immunization was excised immediately after immunization (10 min between immunization and excision) or at 3, 6, 12, or 24 h or after 3, 6, or 9 d after immunization. All mice received adoptively transferred OT-1 cells, which were measured for expansion as in Fig. 1B. It was observed that all mice that had been immunized supported expansion of OT-1 cells on day 10 (i.e., showed persistence), even if the site of immunization had been excised 10 min after immunization (see table below). Mice that did not receive OVA did not support proliferation of OT-1 cells. This rapid time scale of transport of antigen is obviously inconsistent with uptake of OVA by DCs at the site of immunization, which then migrate to the DLN, because this process takes several hours (10, 11). Clearly, that mode of antigen uptake and transport does occur but is not necessary for antigen persistence seen here. Instead, the results in the table are consistent with a rapid transport of OVA through the “conduit system” (11, 12) and suggest that antigen persistence occurs in the resident DCs of the DLN.
Influence of excision of site of immunization on antigen persistence
| Time of excision after immunization | 0 h* | 10 min | 1 h | 3 h | 24 h | Not excised |
| % OT-1 cells proliferated | 1.15 | 77.2 | 84.6 | 83.2 | 89.1 | 83.1 |
Mice were immunized with OVA, and the site of immunization was excised at various time points indicated, as described in Materials and Methods. Ten days after immunization, 5 × 105 CFSE-labeled CD45.1 OT-1 cells were adoptively transferred. Drainage lymph nodes were harvested after 3 d of OT-1 transfer and labeled with anti-CD45.1 and CD8 antibodies, and proliferation of transferred cells was analyzed by flow cytometry.
*The 0 h time point refers to mice immunized with phosphate-buffered saline (PBS) instead of OVA. The earliest time point at which mice can be excised after immunization is 10 min because of the logistics of excision. Hence, the negative controls (PBS-immunized mice) are depicted at the 0 h time point.
Epitope Persistence in Dendritic Cell Cultures in Vitro.
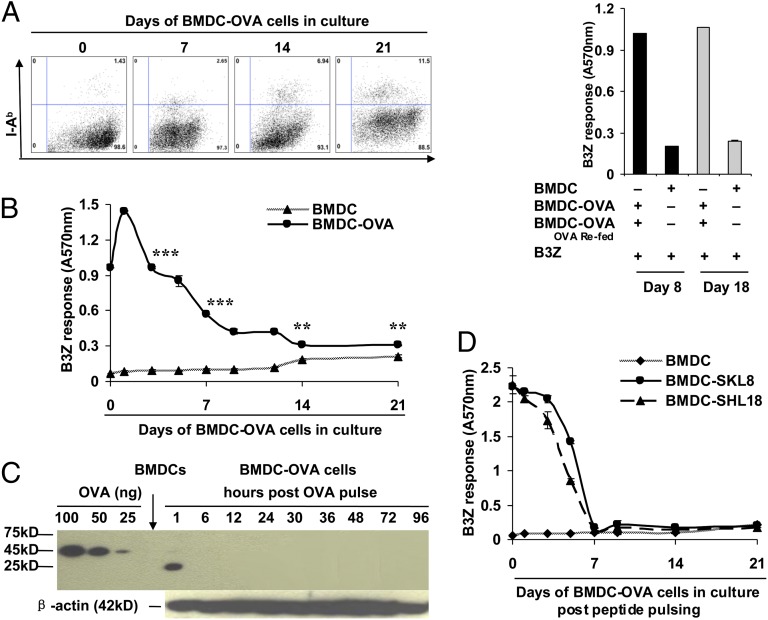
Bone marrow-derived DCs (BMDCs) from C57BL/6 mice were fed lipopolysaccharide-free soluble OVA in culture (such cells referred to henceforth as BMDC-OVA cells), and the viability of the culture was followed. To our surprise, the BMDC-OVA cells remained viable in culture until at least day 21. There was little expansion and little death in culture, such that the cell numbers remained mostly constant over this period. The cells continued to exclude SYTOX Green and did not stain for Annexin V. The CD11c and MHC II expression on the cells remained constant over this time, although MHC II expression was slightly enhanced on days 14 and 21 (Fig. 2A, Left). As a control for viability and functional activity, the BMDC-OVA cells in culture on days 8 or 18 could be refed with OVA, and such OVA-fed cells could stimulate B3Z cells to an equivalent degree (Fig. 2A, Right). The ability of the BMDC-OVA cells to stimulate B3Z cells was investigated at various time points (on days 1, 2, 4, 6, 7, 10, 12, 14, and 21) after feeding of OVA. The results (Fig. 2B) show that the DCs stimulated B3Z cells robustly until day 12, and even at day 21, a statistically significant difference was noted between unpulsed and OVA-pulsed cells. This assay in vitro thus captured the phenomenon of antigen persistence, seen so robustly in vivo, as in Fig. 1B.
Fig. 2.
Immunologic activity of OVA persists in dendritic cells in vitro for several weeks after antigen uptake. (A, Left) Fluorescence-activated cell sorter plots showing the expression levels of CD11c and I-Ab on BMDC-OVA cells cultured in vitro for 1, 7, 14, or 21 d. (A, Right) Kb/SIINFEKL presentation by BMDC-OVA cells in culture 7 d after refeeding with OVA on day 8 (black bars) or day 18 (gray bars) of culture. (B) Antigen persistence in BMDC-OVA cells as tested by the B3Z assay at various time points indicated. **P < 0.01, ***P < 0.001. (C) OVA protein or fragments and β-actin were detected by immunoblotting in cultured BMDC-OVA cells at time points indicated. (D) Duration of Kb/SIINFEKL presentation at time points indicated by BMDC pulsed with SKL8 (SIINFEKL) or SHL18 (LEQLKSIINFEHLKEWTS) on day 0. **P < 0.01, ***P < 0.001. Error bars indicate mean ± SD. The experiments were repeated three to five times.
Persistence of biochemically detectable OVA in the BMDC-OVA cells was followed in vitro. The cells were sampled by immunoblot analysis for various time points after feeding of OVA; it was noted (Fig. 2C) that OVA was degraded within the DCs at the very first time point tested, as seen by the small molecular mass of the detectable fragment (1 h after feeding), and even this degraded fragment was undetectable by the polyclonal antibody at any subsequent point. As a control, endogenously synthesized β actin was detectable in these same lysates at all time points tested.
Size Requirement for Epitope Persistence.
The ability of DCs to sequester other forms of the OVA epitope was tested. BMDCs were pulsed with the precise Kb-binding OVA epitope SIINFEKL, or an extended 18-mer version of it, LEQLKSIINFEKLKEWTS. Washed, pulsed BMDCs in culture were used to stimulate B3Z cells. It was observed that in contrast to intact OVA protein, DCs pulsed with the precise SIINFEKL epitope or its 18-mer variant lost the ability to stimulate B3Z cells by day 7 after antigen exposure (Fig. 2D). This observation suggests that functional persistence of OVA requires some minimal molecular size (and conformation) yet to be determined.
Existence of an Internal Pool for Epitope Precursors.
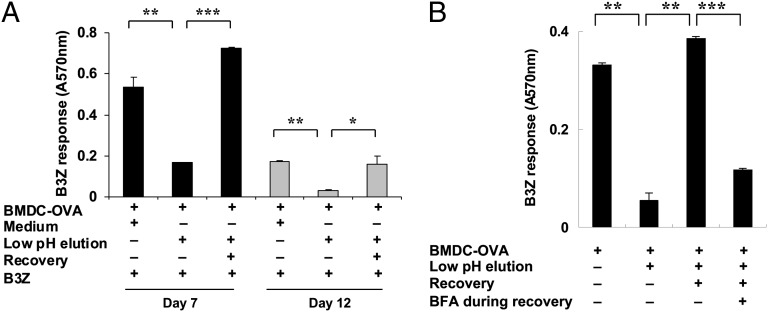
Persistence of OVA in an immunologically active form, in the absence of detectable OVA protein or a large fragment of OVA protein, suggests the existence of an internal pool of SIINFEKL or its precursors within the DCs. Considering the limited half life of Kb/SIINFEKL complexes, such MHC I-peptide complexes are an unlikely source of such a pool. Indeed, experiments testing the half-life of Kb/SIINFEKL complexes on BMDCs pulsed with SIINFEKL, and followed with the 25-D1.16 antibody, confirmed that such complexes disappear within 2 d after pulsing. To detect the presence of an alternate pool, BMDC-OVA cells on day 7 after OVA pulse (well beyond the 1 h postpulse time window when an OVA fragment is biochemically detectable as in Fig. 2C) that were fully capable of engaging B3Z cells were stripped of their surface MHC I-bound peptides by a 45 s exposure to citrate–phosphate buffer pH 3.1 (13). Such treatment resulted in a highly significant loss of surface Kb/SIINFEKL complexes, as seen by a highly reduced ability of the cells to engage B3Z cells (Fig. 3A). However, when these same BMDC-OVA cells were allowed to recover for 20 h without additional exposure to new OVA, their ability to engage B3Z cells recovered to the original extent. These recovered cells could again be stripped as before, and they lost the ability to engage B3Z cells. However, after recovery, on day 12 after pulse, these stripped cells could again engage B3Z cells successfully. If the acid-stripped cells were allowed to recover in the presence of Brefeldin A, the recovery was completely abrogated (Fig. 3B). These experiments reveal the definitive existence of an internal pool of SIINFEKL-containing peptides within the DCs.
Fig. 3.
Sustained Kb/SIINFEKL presentation from an internal epitope pool in BMDCs. (A) BMDCs were fed with soluble OVA on day 0, and the BMDC-OVA cells were cultured for 7 d (black bars) or 12 d (gray bars); their ability to activate B3Z T cells was assessed on days 7 and 12, each time after pulsing the cells with medium or directly after a 45-s exposure to a citrate–phosphate buffer (pH 3.1) or after low-pH exposure and recovery (20 h), as described in Materials and Methods. Unpulsed BMDC were used to stimulate B3Z cells as a negative control, and the background level of stimulation observed was deducted from the numbers obtained for the other groups shown here. (B) Acid-stripped cells, as in A, were allowed to recover in the absence or presence of Brefeldin A, as described in Materials and Methods, and were used to stimulate B3Z cells, as in A. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate mean ± SD. A representative of three independent experiments is shown.
Hsp90 Dependence of Epitope Persistence.
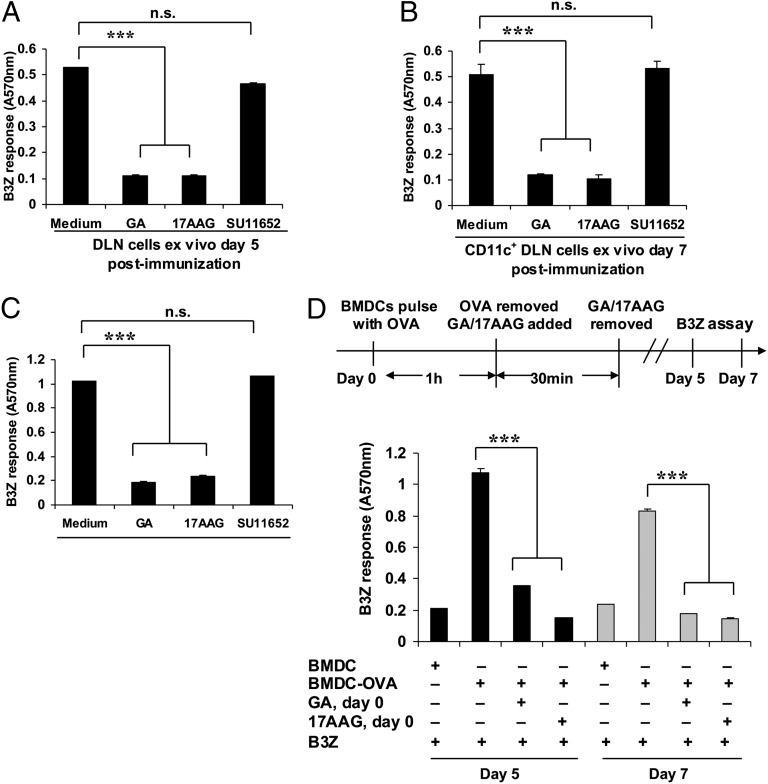
Heat shock proteins (HSPs) and, specifically, hsp90 molecules have been shown to chaperone precursors of SIINFEKL and other epitopes in nonantigen-presenting cells by independent groups (14–17). The possibility that DCs may sequester SIINFEKL precursors as hsp90-peptide complexes was next investigated. C57BL/6 mice were immunized with soluble OVA as before, and DLN cells isolated 5 d after immunization. These cells could stimulate B3Z cells vigorously (Fig. 4A). Treatment of the LN cells ex vivo with the hsp90 inhibitors geldanamycin (GA) or 17-allylamino-17-demethoxygeldanamycin (17-AAG) (18) for 60 min abolished this stimulatory capacity (P < 0.001 each for GA and 17-AAG). (Inhibition of hsp90 under the conditions used does not affect cell viability or synthesis of MHC I.) Because a large number of cellular processes occur through interaction of client proteins with hsp90 and the tyrosine kinases are among a major class of these client proteins, a broad-spectrum tyrosine kinase inhibitor SU11652 was tested. SU11652 did not inhibit cross-presentation of SIINFEKL by the DCs (Fig. 4A). Nonetheless, the possibility that inhibition of hsp90 may be inhibiting cross-presentation and antigen storage through other indirect effects clearly exists and is not ruled out. The experiment shown in Fig. 4A was reproduced in its entirety and quantitatively when repeated with CD11c-positive cells of the draining DLNs, as opposed to whole DLNs (Fig. 4B). A nearly identical requirement of hsp90 was seen in vitro using BMDC-OVA cells (Fig. 4C). Although these results clearly show a requirement for hsp90 in cross-presentation (as in refs. 8 and 10), they do not test if this requirement is involved in epitope sequestration. It was reasoned that if hsp90 is sequestering SIINFEKL precursors, a brief treatment of the BMDCs with hsp90 inhibitors purely at the time of antigen pulse should interfere with generation of an hsp90-peptide pool, such that no persistence is seen. Hence, BMDCs were pulsed with OVA, and immediately after the pulse, they were treated (or not) with GA or 17-AAG for 30 min and washed and cultured for several days. On day 5, BMDCs that had received the OVA pulse but had not been hsp90 inhibited, were tested for their ability to stimulate B3Z cells. They did so vigorously; however, when BMDCs that had been OVA pulsed and hsp90 inhibited solely for 30 min initially were tested on day 5 or day 7 for stimulation of B3Z cells, they were unable to do so (Fig. 4D).
Fig. 4.
Persistence of Kb/SIINFEKL presentation is attenuated by inhibition of hsp90. (A) Stimulation of B3Z cells by draining lymph node (DLN) cells [day 5 after OVA immunization] treated ex vivo and immediately before the assay, with or without geldanamycin (GA) (25 μM), 17-AAG (50 μM), or SU11652 (1 μM). (B) Stimulation of B3Z cells by purified CD11c positive cells from DLNs (day 7 after OVA immunization) treated ex vivo and immediately before the assay, with or without GA, 17-AAG, or SU11652, as in A. (C) Stimulation of B3Z cells by BMDC-OVA cells (day 5 after OVA pulsing) treated in vitro and immediately before the assay, with or without GA, 17-AAG, or SU11652, as in A. (D) Stimulation of B3Z cells by BMDC-OVA cells, day 5 (black bars) or day 7 (gray bars) after OVA treated, in vitro for 30 min, only on day 0 and immediately after OVA-uptake, with or without GA (25 μM) or 17-AAG (50 μM). In contrast to A and B, no inhibitors were used immediately before the assay. ***P < 0.001. Error bars indicate mean ± SD. A representative of three independent experiments is shown.
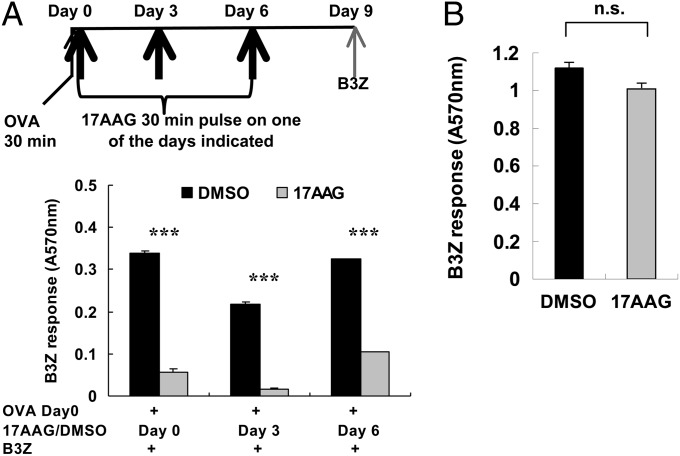
To dissect antigen presentation from antigen storage more thoroughly, we designed an experiment to separate the OVA pulse, the inhibition of hsp90, and the readout of antigen persistence (B3Z stimulation assay) chronologically from each other so that any effects of inhibition of hsp90 on antigen presentation by DCs (17) would not interfere with interpretation of the data (Fig. 5A, Upper). BMDCs were pulsed with OVA for 30 min on day 0 and were also exposed to 17-AAG (or as a control, dimethyl sulfoxide) for 30 min on day 0 itself (immediately after the OVA pulse) or on day 3 or 6. All cultures were read for B3Z stimulation on day 9. Here, the OVA pulse is separated from hsp90 inhibition by 0, 3, or 6 d, and hsp90 inhibition is separated from the readout of cross-presentation by 3, 6, or 9 d. The results (Fig. 5A, Lower) show that a 30 min pulse with 17-AAG at any of the times on day 0, 3, or 6 results in highly significant inhibition of cross-presentation as measured on day 9. As a control, it was observed (Fig. 5B) that 17-AAG–treated cells can cross-present OVA efficiently if OVA is provided 3 d after the 17-AAG pulse. Because previous reports have shown that inhibition of hsp90 immediately before OVA pulse inhibits cross-presentation (17), the result in Fig. 5B indicates that the direct inhibitory activity of 17-AAG (on cross-presentation) is lost within 3 d. This result contrasts with the long-lasting effect of 17AAG on antigen storage shown here.
Fig. 5.
Role of hsp90 in antigen sequestration vs. antigen presentation. (A) BMDC cultures were pulsed with OVA for 30 min, followed by a 30-min pulse with 17AAG or dimethyl sulfoxide (as a control) on day 0 (immediately after OVA pulse), or on days 3 and 6 after OVA pulse. All cultures were assayed for B3Z stimulation on day 9 after OVA pulse. (B) BMDC cultures were treated with 17-AAG for 30 min, washed, and placed back in culture. A 30-min pulse of OVA was added on day 3, and the culture was assayed for B3Z stimulation on day 4. ***P < 0.001. Error bars indicate mean ± SD.
Discussion
Altogether, our results show that DCs in vivo and DC cultures in vitro can sequester the Kb-binding epitope of OVA or its precursors, but not the intact antigen nor a large fragment thereof, in an hsp90-dependent manner for long periods, in the order of several weeks. The evidence suggests strongly that the DLN-resident DCs are the site of antigen storage, although other sites of storage are not excluded. The release of antigen from the intracellular storage site appears to occur in a continuous manner, and the antigenic moiety must transit through the endoplasmic reticulum en route to loading onto MHC I molecules. Sequestration of antigens by DCs has been shown in a small number of recent studies (19–22), and our results extend those findings by providing the beginnings of a mechanistic basis for some aspects of those earlier results. Faure et al. (20) and Reboulet et al. (21) demonstrated antigen persistence in vitro in DCs pulsed with OVA-derived peptides or OVA-expressing cells. In studies that show antigen persistence in vivo, mice were immunized with OVA in the presence of anti-OVA antibody or with DCs pulsed in vitro with antibody-bound OVA (19, 22). Our results substantially extend these earlier studies by demonstrating antigen persistence in mice immunized with soluble OVA in vivo, as well as in DCs pulsed with soluble OVA in vitro (in the absence of facilitating mechanisms such as anti-OVA antibodies), and further advance the idea of a truly long-term (4 wk or longer) internal antigen depot within the DCs in vivo. Finally, our results uncover a discrete aspect of the mechanism of long-term antigen storage by showing that hsp90, which has been implicated in direct and indirect antigen presentation before (14–17), plays a specific role in antigen persistence as well.
A number of studies have shown that viral antigens persist in the host long after the infection is cleared. This is true for chronically infecting viruses, such as the respiratory syncytial and Sendai viruses, as well as lytic influenza viruses (as cited in the introductory paragraphs of this article). The evidence that antigens persist comes from immunologic rather than biochemical studies: typically, it has been shown by the ability of the host, which has cleared the infection and in which no antigen is detectable, to readily support stimulation of adoptively transferred naïve T cells for weeks after the infection has been cleared. It has been reasonably assumed that the genetic information of the virus is somehow preserved in the host, and its continued expression at a low level is responsible for this persistence. Indeed, viral transcripts have now been detected long after the virus has been cleared; nonetheless, the antigen appears to persist immunologically even longer after such transcripts cease to be detectable (8). Thus, the molecular basis of antigen persistence has remained mysterious. Our studies provide a molecular mechanism of such long-term antigen persistence in the absence of any genetic information for the antigen. Because antigen persistence is linked with the nature of T-cell memory elicited in response to infection (8, 9), our observations open a window into an understanding and manipulation of memory responses as well.
Because DCs have a limited half-life in vivo (23–25), our results invoke, of necessity, transfer of the antigen depots from one antigen-presenting cell to another. Such a transfer has been invoked previously (26), and our results provide a handle on that question by perhaps identifying the nature of the transferred material from one antigen-presenting cell to another. The present results raise a number of new questions as well. What is the cell biologic basis of the uniqueness of DCs with respect to sequestration? This issue is particularly curious, because hsp90 is present in DCs as well as other cells and hsp90-peptide complexes have been demonstrated in non-DCs (as cited above). Are some DC subsets better than others at sequestering epitopes? Are the hsp90-peptide depots located in a specific intracellular compartment? Dendritic cell aggresome-like–induced structures within DCs have been shown to act as sites of antigen storage; however, DC aggresome-like–induced structures acquire newly synthesized antigen, and they protect antigen for several hours rather than weeks (27) and are therefore unlikely sites of sequestration. These questions require further experimental attention and promise to uncover significant new mechanisms in adaptive immunity.
The observation that antigen sequestration occurs through the agency of an abundant protein, hsp90, whose expression is modulated by a range of physiologic conditions, including stress, fever, infection, and aging (28), adds a unique level of regulation on antigen sequestration and hence on most aspects of adaptive immune response to infections, cancers, and self-antigens.
Materials and Methods
Mice.
Female C57BL/6 mice, 6–12 wk of age, were purchased from Jackson Laboratory. Female OT-I TCR transgenic mice were obtained from Taconic. All animal works were approved by the Center for Laboratory Animal Care of University of Connecticut School of Medicine.
Cells.
B3Z T-cell hybridomas were provided by Nilabh Shastri (University of California, Berkeley, CA). BMDCs were generated from C57BL/6 mice. The bone marrow was flushed out from the femurs and tibiae and cultured in complete RPMI 1640 with 10% (vol/vol) fetal calf serum and 20 ng/mL granulocyte-macrophage colony-stimulating factor (Thermo Scientific). Immature BMDCs were used in experiments on day 6 or 8.
Pulse Loading of BMDCs with OVA, SKL8, and SHL18.
Washed BMDCs were incubated at 37°C for 1 h with 10 mg/mL lipopolysaccharide-free OVA protein or OVA peptides SKL8 (SIINFEKL) and SHL18 (LEQLKSIINFEHLKEWTS) containing equal amounts of SIINFEKL epitopes with OVA (Genemed). To remove free antigen, BMDCs were washed three times with medium and subsequently cultured antigen free for the periods indicated. The expression of CD11c, major histocompatibility complex II, Kb/SIINFEKL complexes on the cells was quantitated by fluorescence-activated cell sorter using CD11c, I-Ab, and 25-D1.16 Abs (Pharmingen).
In Vivo Antigen Presentation Assay.
C57BL/6 mice were immunized intradermally and then adoptively transferred with CFSE-labeled OT-I CD8-positive T cells at different time intervals (Fig. 1A) or 1 d later after each immunization (Fig. 1D). Three days after each OT-I transfer, drainage lymph nodes were harvested. The drainage lymph node cells were collected and incubated with anti-CD16/32 at 4°C for 10 min, then stained with allophycocyanin-labeled anti-CD8 Ab at 4°C for 30 min and phycoerythrin-labeled Kb/SIINFEKL pentamer for 10 min at room temperature. Assessment of proliferation was made using CFSE dilution of the population gated on live, CD8-positive Kb/SIINFEKL pentamer-positive cells. All cells that had diluted CFSE at least one time were regarded as proliferating cells. For experiments involving excision of the site of immunization, mice were anesthetized and the area of injection surgically removed and sutured. After 10 d, 5 × 105 CFSE-labeled CD45.1 OT-1 cells were adoptively transferred. Drainage lymph nodes were harvested after 3 d and labeled with anti-CD45.1 and CD8 antibodies; proliferation of transferred cells was analyzed.
Purification of Total and CD11c-Positive and CD11c-Negative Cells from Drainage Lymph Nodes.
Drainage lymph nodes were digested at 37°C for 30 min in RPMI supplemented with 1 mg/mL of type II and IV collagenase also containing 50 Kunitz units/mL of DNase (Sigma). After incubation, EDTA was added to a final concentration of 5 mM and incubated for an additional 5 min. Cells were collected and washed with MACS buffer (Miltenyi Biotec) (phosphate-buffered saline with 0.5% fetal bovine serum and 2 mM EDTA, pH 7.2). Cells were incubated with CD11c microbeads (Miltenyi Biotec) for 15 min at 4°C under slow rotation. Selection of microbead-bound cells was performed according to the manufacturer’s instructions.
Low pH Elution of BMDC-OVA cells.
Bone marrow-derived OVA cells were incubated for 45 s with mild acid citrate–phosphate buffer (pH 3.1) at room temperature to disrupt major histocompatibility complex I-bound peptides (13). Cells were either fixed in 0.2% paraformaldehyde directly after elution or incubated in culture medium for recovery at 37°C with or without 1.5 μg/mL Brefeldin A (eBioscience) for 6 h and then with 0.75 μg/mL for overnight and then washed and fixed.
Acknowledgments
This work was supported by National Institutes of Health Grant CA084479 (to P.K.S.), Northeastern Utilities Chair in Experimental Oncology (P.K.S.), and the China Scholarship Council (C.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.P’ringle C-R, Shirodaria P-V, Cash P, Chiswell D-J, Malloy P. Initiation and maintenance of persistent infection by respiratory syncytial virus. J Virol. 1978;28:199–211. doi: 10.1128/jvi.28.1.199-211.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwarze J, O’Donnell D-R, Rohwedder A, Openshaw P-J. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169:801–805. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- 3.Mori I, et al. Parainfluenza virus type 1 infects olfactory neurons and establishes long-term persistence in the nerve tissue. J Gen Virol. 1995;76:1251–1254. doi: 10.1099/0022-1317-76-5-1251. [DOI] [PubMed] [Google Scholar]
- 4.Jelley-Gibbs D-M, et al. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med. 2005;202:697–706. doi: 10.1084/jem.20050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zammit D-J, Turner D-L, Klonowski K-D, Lefrançois L, Cauley L-S. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner DL, Cauley LS, Khanna KM, Lefrançois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna K-M, et al. In situ imaging reveals different responses by naïve and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur J Immunol. 2008;38:3304–3315. doi: 10.1002/eji.200838602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim T-S, Hufford M-M, Sun J, Fu Y-X, Braciale T-J. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazilleau N, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol. 2007;8:753–761. doi: 10.1038/ni1472. [DOI] [PubMed] [Google Scholar]
- 10.Itano A-A, Jenkins M-K. Antigen presentation to naive CD4 T cells in the lymph node. Nat Immunol. 2003;4:733–739. doi: 10.1038/ni957. [DOI] [PubMed] [Google Scholar]
- 11.Itano A-A, et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 12.Sixt M, et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity. 2005;22:19–29. doi: 10.1016/j.immuni.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Storkus W-J, Zeh H-J, 3rd, Salter R-D, Lotze M-T. Identification of T-cell epitopes: rapid isolation of class I-presented peptides from viable cells by mild acid elution. J Immunother Emphasis Tumor Immunol. 1993;14:94–103. [PubMed] [Google Scholar]
- 14.Ishii T, et al. Isolation of MHC class I-restricted tumor antigen peptide and its precursors associated with heat shock proteins hsp70, hsp90, and gp96. J Immunol. 1999;162:1303–1309. [PubMed] [Google Scholar]
- 15.Kunisawa J, Shastri N. Hsp90alpha chaperones large C-terminally extended proteolytic intermediates in the MHC class I antigen processing pathway. Immunity. 2006;24:523–534. doi: 10.1016/j.immuni.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Callahan M-K, Garg M, Srivastava P-K. Heat-shock protein 90 associates with N-terminal extended peptides and is required for direct and indirect antigen presentation. Proc Natl Acad Sci USA. 2008;105:1662–1667. doi: 10.1073/pnas.0711365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imai T, et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci USA. 2011;108:16363–16368. doi: 10.1073/pnas.1108372108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerji U, Judson I, Workman P. The clinical applications of heat shock protein inhibitors in cancer - present and future. Curr Cancer Drug Targets. 2003;3:385–390. doi: 10.2174/1568009033481813. [DOI] [PubMed] [Google Scholar]
- 19.van Montfoort N, et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc Natl Acad Sci USA. 2009;106:6730–6735. doi: 10.1073/pnas.0900969106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faure F, et al. Long-lasting cross-presentation of tumor antigen in human DC. Eur J Immunol. 2009;39:380–390. doi: 10.1002/eji.200838669. [DOI] [PubMed] [Google Scholar]
- 21.Reboulet R-A, Hennies C-M, Garcia Z, Nierkens S, Janssen E-M. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J Immunol. 2010;185:3337–3347. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCloskey M-L, Curotto de Lafaille M-A, Carroll M-C, Erlebacher A. Acquisition and presentation of follicular dendritic cell-bound antigen by lymph node-resident dendritic cells. J Exp Med. 2011;208:135–148. doi: 10.1084/jem.20100354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamath A-T, Henri S, Battye F, Tough D-F, Shortman K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood. 2002;100:1734–1741. [PubMed] [Google Scholar]
- 24.Geissmann F. The origin of dendritic cells. Nat Immunol. 2007;8:558–560. doi: 10.1038/ni0607-558. [DOI] [PubMed] [Google Scholar]
- 25.Woodland D-L, Kohlmeier J-E. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat Rev Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 26.Allan R-S, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Lelouard H, et al. Transient aggregation of ubiquitinated proteins during dendritic cell maturation. Nature. 2002;417:177–182. doi: 10.1038/417177a. [DOI] [PubMed] [Google Scholar]
- 28.Taipale M, Jarosz D-F, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]