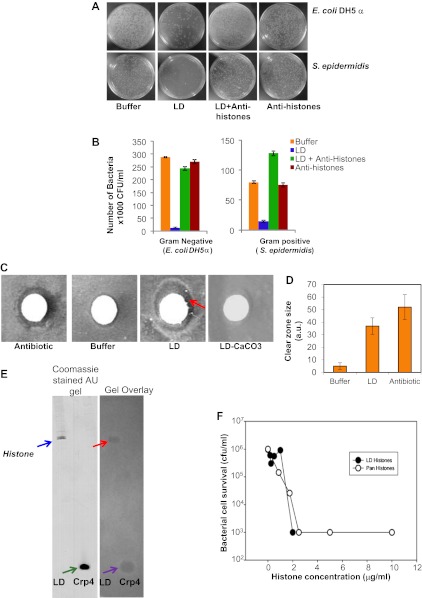
Figure 1. LDs kill bacteria via droplet bound histones.
(A). Representative plates in a colony forming assay, showing growth of Gram-negative (Escherichia coli DH5α, top) and Gram-positive (Staphylococcus epidermidis, bottom) bacteria, where a known amount of bacteria were incubated at 37°C either in buffer alone, or with LDs pre-treated with or without anti-histone antibodies. In buffer (left, ‘Buffer’), many colonies (white spots) were observed, but in the presence of LDs (LD), the observed number of colonies was greatly decreased, demonstrating an antibacterial effect of the LDs. Pre-treatment of the droplets with anti-histone antibodies abolished this effect (LD + Anti-histones). (B). Quantification of colony forming assay in A. Each bar represents the mean number of observed colonies, in three independent trials, presented with the standard error. (C). Disc diffusion assay over a lawn of E. coli DH5α. A potential antibacterial agent is placed on a small sterile piece of filter paper (white circle); a cleared area (darker region) indicates antibacterial activity. Positive control: the antibiotic kanamycin (Antibiotic); growth inhibition region indicated by the red arrow. Negative control: buffer (Buffer). LDs isolated in the presence (LD-CaCO3) or absence (LD) of alkaline carbonate were spotted on sterile discs; bacterial inhibition was observed in the untreated droplets (LD) but not in the carbonate-treated droplets (LD-CaCO3). The filter papers are 7 mm in diameter. (D). Quantification of the size of the clear zone in the disc diffusion assay from C. Fifteen independent disc diffusion assays were performed with purified LDs from Drosophila embryos, the antibiotic kanamycin, or buffer. Antimicrobial activity of compounds was quantified as the diameter of the clear zones surrounding the filter papers after subtraction of filter papers diameter (1 A.U. = 0.1 mm). (E). Use of a gel-overlay assay to determine the identity of the anti-bacterial protein(s) on the LDs. Proteins extracted from LDs (LD, left lane) were run in duplicate on an AU-gel; murine cryptdin 4 (Crp 4, right lane) served as positive control. After electrophoresis, the gel was split. One half (left) was stained by Coomassie Blue; the histone bands are indicated by a blue arrow and the crp 4 control is indicated by the green arrow. The other half (right) was used in a gel overlay assay (see ‘Materials and methods’) to reveal regions of the gel able to inhibit bacterial growth (inhibition by LD is indicated by the red arrow and that by crp 4 control is indicated by the violet arrow). Inhibition of bacterial growth due to proteins on the LDs was only observed in a single region, corresponding to the histones (red arrow). Consistent with this, mass spectrometry of proteins cut from the Coomassie gel corresponding to the killing region identified predominantly histones H2A and H2B (see ‘LDs have antimicrobial activity’). (F). E. coli ML35 cultures were transiently (∼1 hr) exposed to commercial calf thymus pan-histone proteins (Sigma) or gel-extracted LD-histones (from the gel-overlay assay). Both preparations show similar potency for bacterial killing.