Table 2.
Thermodynamic Cycle to Calculate 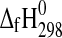 (MgSO4·nH2O), n=3, 4
(MgSO4·nH2O), n=3, 4
| Reaction | Enthalpy (kJ·mol−1) | |
|---|---|---|
| 1 | α-MgSO4 (cr)=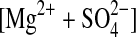 (aq) (aq) |
−85.52a±0.91b (3)c |
| 2starkeyite | MgSO4·4H2O (cr)=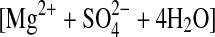 (aq) (aq) |
−18.45±0.17 (5) |
| 2trihydrate | MgSO4·3H2O (cr)=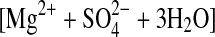 (aq) (aq) |
−21.01±0.79 (2) |
| 3 | H2O (l)=H2O (aq) | 0 |
| 4 | Mg (cr) + S (cr) + 2O2 (g)=α-MgSO4 (cr) | −1288.5±0.5d |
| 5 | H2 (g) + ½O2 (g)=H2O (l) | −285.8±0.1e |
| 6starkeyite | Mg (cr) + S (cr) + 4O2 (g) + 4H2 (g)=MgSO4·4H2O (cr) | −2498.7±1.1 |
| 6trihydrate | Mg (cr) + S (cr) + 3.5O2 (g) + 3H2 (g)=MgSO4·3H2O (cr) | −2210.3±1.3 |
