Abstract
Distortions in time perception and timed performance are presented by a number of different neurological and psychiatric conditions (e.g. Parkinson's disease, schizophrenia, attention deficit hyperactivity disorder and autism). As a consequence, the primary focus of this review is on factors that define or produce systematic changes in the attention, clock, memory and decision stages of temporal processing as originally defined by Scalar Expectancy Theory. These findings are used to evaluate the Striatal Beat Frequency Theory, which is a neurobiological model of interval timing based upon the coincidence detection of oscillatory processes in corticostriatal circuits that can be mapped onto the stages of information processing proposed by Scalar Timing Theory.
Keywords: time perception, timing, striatum, frontal lobe, Parkinson's disease
Introduction
Our subjective sense of time is fundamental to our psychology and conceptions of reality, and is part of the intellectual structure by which we make sense of the temporal course of events in our lives. Pathophysiological distortions in human timing and time perception are of interest to both basic and clinical researchers for several reasons. Basic scientists attempting to delineate the psychological mechanisms mediating ‘normal’ timing in the seconds-to-minutes range are particularly interested in patients who have a known neuropathology that mirrors certain pharmacological and neurological manipulations in laboratory animals (e.g. impaired basal ganglia function and altered levels of the brain neurotransmitter dopamine, as in Parkinson's disease—see Meck, 1996, 2005, 2006a, b; Buhusi and Meck, 2005; Merchant et al., 2008a; Zarco et al., 2009; Coull et al., 2011), in the expectation that human neurobiological mechanisms of timing might be better elucidated. To this end, we will discuss how reported pathophysiological findings may inform the development of neurobiological models of interval timing (Matell and Meck, 2000, 2004; Matell et al., 2003, 2011; Meck et al., 2008). From a clinical perspective, examinations of timing ability in patients with certain psychiatric or behavioural disorders—particularly those that are (at least in part) defined by characteristic changes in the apparent temporal organization of cognition or behaviour [e.g. attention-deficit hyperactivity disorder (ADHD), autism and schizophrenia]—may help to ameliorate understanding of the psychological experience of these disorders and their potential remediation. In this regard, we will discuss how pathophysiological distortions in timing and time perception in the seconds-to-minutes range may improve our understanding of certain psychiatric, developmental and childhood disorders, as well as behavioural and cognitive tendencies (e.g. impulsivity). It is useful to bear in mind that there is no human clinical condition that can be defined solely as a disorder of timing and time perception per se (in fact, a complete inability to estimate time is likely incompatible with life). However, distortions and perturbations in timing ability are present, to varying degrees, in many patient populations, and may or may not accompany differences in other aspects of sensory processing, as well as developmental, cognitive and behavioural profiles (Meck and Williams, 1997a, b; Brannon et al., 2004, 2008; Balci et al., 2009; Allman, 2011; Allman et al., 2011b).
It appears that when humans and other animals ‘know in advance’ that they are required to time something (prospective timing), they are sensitive to and can remember the temporal aspects of and between events (e.g. how ‘long’ a stimulus is present, and the time ‘between’ its successive presentations), and can utilize temporal knowledge to mediate their expectations and behaviour (learning and conditioning). In addition to these qualities, humans are also ordinarily able to discriminate ‘time after the fact’ (e.g. they can estimate how ‘long’ an event lasted even though explicit timing was not required during the event itself; i.e. retrospective timing), can orient themselves in time and have a sense of past, present and future (temporal perspective). The emphasis of this review is to report those findings characterized from an information processing approach (developed in animal models), which is grounded within the study of the relative similarities (and phylogenic generality) to human timing, but which was not specifically designed to account for retrospective timing or temporal perspective taking; which typically requires higher order processes involving a concept of temporal order, including an understanding of past, present and future (Roberts, 2002; Block, 2003; Grondin, 2010; Piras and Coull, 2011). Our rationale is that using this information processing approach is, among other things, particularly informative with regard to pinpointing ‘where’ any differences in timing ability reside (rather than just revealing ‘what’ the differences are), which in turn facilitates a better understanding of diagnosing the likely psychological and neurobiological processes that are responsible for producing individual differences and/or pathophysiological distortions of time—accordingly, interval-timing models have been accredited as being the most successful in the whole of psychology in this regard (Wearden, 2001). Hence the findings highlighted in this review should not be considered a complete account of all pathophysiological timing distortions (and due to the fact that motor programme and interval-timing mechanisms are frequently posited to have a separable neural basis, we avoid studies employing tasks in which these processes cannot be easily separated) (Madison, 2001; Spencer et al., 2003; Gooch et al., 2007; Levit-Binnun et al., 2007; Bueti et al., 2008). Nevertheless, towards the end of this review, we will extend our discussion of pathophysiological differences to include other aspects of timing (particularly temporal processing of sensory information and temporal perspective taking), as it seems likely that a primitive sensitivity to duration (and ‘sister’ abilities such as simultaneity and temporal order) may form at least part of the basis for successful development and maintenance of ‘normal’ higher order notions of time and temporality (Fraisse, 1982). In fact, from a developmental perspective, a sensitivity to perceive and respond to duration might be inextricably linked to the development and maintenance of ‘higher order’ processes that are defined by a temporal dimension i.e. planning, attention and working memory (Lustig et al., 2005; Allman and DeLeon, 2009; Grondin, 2010; Allman, 2011) and even theory of mind (Baron-Cohen, 2001; Nelson, 2001; Nelson et al., 2003).
Before reviewing pathophysiological findings per se, we consider it useful to couch our forthcoming discussion with a brief description of the background theoretical framework and methodology that is heavily recruited throughout this review. This preamble seeks to ‘set the context’ for how pathophysiological findings might be evaluated and interpreted.
An information processing model of interval timing
Perhaps one of the most perplexing issues surrounding our subjective experience of time is that there is no dedicated sense organ for duration, as there is for other senses (yet time is commonly referred to as being perceived). Usually, investigators of sensory perception adopt a psychophysical approach that can be defined as an attempt to quantify the sensory response to physical stimuli (see Gescheider, 1997, for a complete review) and this approach has also been applied to the study of time. Despite the fact that time is not a stimulus in the usual sense of the term, the experience of duration in the seconds-to-minutes range has accordingly been found to share many properties and follow the same mathematical rules and principles as perception by other senses such as hearing and vision (Gibbon, 1991; Gibbon et al., 1997; Eisler et al., 2008; Merchant et al., 2008b; Lejeune and Wearden, 2009; Grondin, 2010; Coull et al., 2011). For instance, we can say that the perception of a difference between (the brightness of) two lights is subliminal (meaning that we cannot perceive the difference) and vision researchers can identify some difference threshold that needs to be exceeded for the discrimination to be made (known as the difference limen; hence sublimen in the former case). Moreover, this perception can be influenced (or changed) by a variety of stimulus and contextual conditions (e.g. it is harder to detect the difference of one extra ‘notch’ of brightness if the bulbs are bright than if they are dim)—as is the case for the experience of time (e.g. a discrimination between 1 versus 3 s, is easier than 61 versus 63 s). Two fundamental properties of ‘normal’ perception have emerged (e.g. for both vision and time, and for timing in humans and other animals): the intensity of the internal perception (sensation) is linearly related to the magnitude of external stimulation (i.e. an objectively longer duration is subjectively perceived as longer); and increases in the magnitude of a physical stimulus produce proportional increases in the variance of the perception (i.e. timing of a longer duration is less precise), which is referred to as scalar variability or Weber's law (Allan, 1991; Lejeune and Wearden, 2006; Zarco et al., 2009). Therefore, any successful conceptual model of interval timing should be capable of accommodating these striking regularities, and other characteristic features [e.g. a given duration is typically perceived as ‘longer’ if it is an auditory compared to visual stimulus, (Goldstone and Lhamon, 1974; Penney et al., 1998, 2000)] and be applicable to methods that enable these properties to be assessed (some of these methods are described below).
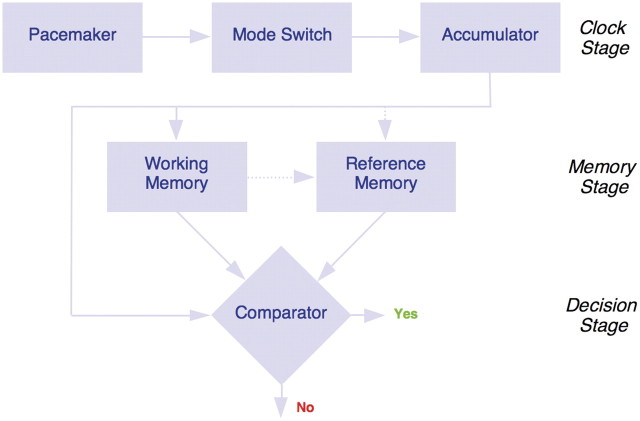
Arguably the most influential timing theory, Scalar Expectancy Theory—also referred to as Scalar Timing Theory—divides the temporal processing system into clock, memory and decision stages, as illustrated in Fig. 1 (Gibbon et al., 1984; Meck, 1984; Matell and Meck, 2000). Essentially, it is assumed that, during the onset of a ‘to-be-timed’ signal, a switch (controlled by attention) closes and allows pacemaker pulses (or ‘clock ticks’) to be collected into an accumulator; this pacemaker-switch-accumulator is the ‘perceptual’ (or ‘clock’) component of the system. If after some time (and hence, arbitrary number of accumulated pulses), the corresponding signal duration acquires some added significance (e.g. it is followed by the delivery of feedback or indicates some other change in the environment), the contents of the accumulator (held to represent the experienced duration) are transferred from working memory to reference memory for long-term storage. The idea is that when the duration is presented (or experienced) again on another trial, a ratio–decision rule operates if the current contents of the accumulator reach or exceed some threshold of similarity to a randomly selected reference memory of the given duration. Scalar Expectancy Theory holds that reference memory contains a distribution of stored accumulator values or clock readings, and that this distribution is the source of scalar variability (Gibbon and Church, 1984, 1992; Penney et al., 1998, 2000). According to this account, individual and pathophysiological differences in timing and time perception might be attributable to alterations in the function of attention (e.g. time sharing), clock (e.g. pacemaker speed), memory (e.g. encoding and decoding) or decision (e.g. response rule or bias) stages of the system (Meck, 2001, 2006a, b, c; Buhusi and Meck, 2009a, b). To these ends, quantitative models that attempt to simulate aspects of human performance on interval-timing tasks within the conceptual framework of Scalar Expectancy Theory have been developed (for a review see Rakitin et al., 1998; Allan and Gerhardt, 2001; Wearden, 2003). During this additional form of analysis, acquired data can be simulated through computer models and the ‘best fit’ produces various parameters that represent functioning of different aspects of the timing system. Although the scalar property of interval timing could reside at either the clock or memory stage, in Scalar Expectancy Theory the duration is usually assumed to be timed without error (clock stage) with scalar variability attributed to variability in transferring/encoding the clock reading into reference memory (memory stage). Although Scalar Expectancy Theory does allow for variation in clock speed (which could produce the scalar property), inferences about ‘what’ is responsible for producing differences in timing are usually discussed in terms of the quality and extent of variation (or ‘fuzziness’) in reference memory (e.g. whether the duration is stored as proportionally shorter or longer than it actually is), or some form of response bias (Wearden, 2001). In humans and other animals, targeted manipulations designed to affect the relative function of the clock, memory and decision stages described by Scalar Expectancy Theory are interpretable by their characteristic effects on the patterns of variability observed in the obtained timing functions (Meck, 1983; Meck et al., 1985; Matell and Meck, 2000; Buhusi and Meck, 2005; Wearden and Lejeune, 2008).
Figure 1.
The information processing model of interval timing as specified by scalar expectancy theory. Adapted from Gibbon et al. (1984).
Psychophysical methods
It was the instrumental responding initially demonstrated by rats and pigeons trained on fixed-interval schedules of reinforcement [i.e. the first response after a stimulus has been presented for 10 s produces food (Ferster and Skinner, 1957)], coupled with findings from Pavlovian conditioning (Pavlov, 1927) in which animals learn to associate an initially neutral stimulus with the presentation of a biologically pre-potent stimulus (e.g. a light flashes and food is delivered 10 s later) that pioneered the study of interval timing in animals. Since the advent of the field of timing and time perception as we know it today (see Gibbon, 1991; Allan, 1998; Wearden, 2005, for historical overviews), the experience of duration has been studied under a wide range and variety of stimulus and contextual factors, some of which have been studied in certain patient (or at-risk) populations. At the generic level, these procedures can be defined as those that pertain to the study of time perception (a response or temporal judgement ‘is not’ required to be accurately timed), such as temporal bisection and ordinality-comparison procedures. Or they can measure timed performance (a response or temporal judgement ‘is’ required to be accurately timed), such as peak-interval and temporal production/reproduction procedures (see Allan, 1979; Paule et al. 1999; Church, 2003 for descriptions of standard interval-timing procedures).
Temporal bisection and ordinality comparison
In the temporal bisection procedure, a participant is typically trained (with feedback to satisfy some acquisition criterion) to discriminate between two standard ‘anchor’ durations that are signalled by serial (auditory or visual) presentations of the same physical stimulus (e.g. a blue circle) that appears on separate trials for each of the two anchor durations; and is required to classify a temporal judgement as either ‘short’ or ‘long’, by selecting between two different response options (e.g. two different buttons or keys). Following this, participants are then presented with a new set of stimulus durations that are intermediate to (in between) the two anchor durations, and are required to make a judgement about a given duration's ‘similarity’ to the ‘short’ or ‘long’ anchor. Typically, during the so-called ordinality-comparison procedure, there are no pre-trained standards, but rather a pair of stimulus durations is presented in close succession to one another on the same trial, and the second duration is judged relative to the first. Regardless of the timing procedure used, it is the proportion of ‘long(er)’ responses (often denoted as ‘pLong’) for each signal duration (when plotted), which constitutes a psychometric timing function (usually sigmoid curves). Changes in accuracy and precision can be inferred from the horizontal placement and slope of the timing functions, respectively (Meck, 1983).
In temporal bisection, the duration that produces 50% ‘pLong’ responses (when the participant is equally likely to classify the duration as ‘short’ or ‘long’) is known as the bisection point or point of subjective equality and in humans and other animals, the point of subjective equality is usually located near the geometric mean of the two anchor durations (Church and Deluty, 1977; Allan and Gibbon, 1991; Wearden and Bray, 2001); temporal variability is indexed by the difference limen (half of the difference between 75% and 25% ‘pLong’ divided by the point of subjective equality); and sensitivity by the Weber ratio to assess scalar timing (difference limen divided by the point of subjective equality). As Weber ratio values are thus normalized, temporal sensitivity across a range of duration pairs can be reliably compared. The scalar property can also be assessed by plotting the psychometric function obtained with two different duration ranges on the same relative time scale; in fact, they are most often found to superimpose (so it looks as though only one function is present—Church et al., 1994; Rakitin et al, 1998).
Peak-interval procedure and temporal reproduction
During the standard peak-interval procedure, a single ‘target’ duration is repeatedly presented. Subsequently, participants are required to centre a series of responses (e.g. space bar presses) around the time that corresponds to the ‘target’ duration or ‘window of opportunity’ (Malapani et al., 1998; Rakitin et al., 1998). On a temporal reproduction task, the participant is typically required to make some form of response that is equivalent in duration to the target duration (Fortin et al., 2009). Across both timing procedures, averaging across trials typically produces a Gaussian-shaped response function. Accuracy is reflected by the location of the peak of the response distribution, and precision is reflected by the spread of the function. Scalar variability is indexed by the coefficient of variation (CV), which is the standard deviation (spread) divided by the mean (peak time).
Timescale invariance as revealed by psychophysical timing procedures: scalar property and superimposition
Scalar Expectancy Theory (Gibbon, 1977; also known as Scalar Timing Theory—Gibbon et al., 1984; Church, 2003) posits that the temporal control of behaviour is directly related to estimates of the target duration. These estimates are essentially scale transforms of a ‘unit timer’ appropriate to the estimation of one unit of time. Although subjects may differ substantially in the accuracy and precision of their temporal estimates, their timing is similar in that: changes in the value of the time being estimated lead to a simple scale transform of the unit timer. Gibbon (1977) proposed a specific translation mechanism that reflects expectancies of reinforcement or feedback based upon scalar time estimates of when these events are due. Responding in simple timed-based schedules of reinforcement (e.g. peak-interval procedure) is viewed as resulting from discrimination between the current or local expectancy of feedback and the overall expectancy of feedback. Thus the Scalar Expectancy Theory is properly described as a discrimination or threshold theory of temporal control. As indicated by Gibbon (1977, p. 281), ‘the core of the scalar-timing hypothesis is that variance of time estimates increases with the square of the mean, and accordingly, the account focuses on the first two moments of distributional phenomena’. Moreover, in scalar timing, the mean and standard deviation are both proportional to the interval being timed so that the coefficient of variation is held constant across a range of durations (Gibbon et al., 1997—but also see Lewis and Miall, 2009). As further indicated by Gibbon (1977, p. 301), Weber's law in a psychophysical context is typically characterized as constant discriminability with a constant ratio of an increment in stimulation to a standard stimulus. The result is that the psychometric functions relating discriminability to the different target durations being compared should superimpose when plotted as a function of the ratio of the stimulus values. As a consequence, Weber's law requires both a ratio comparison and the scalar assumption in order to produce the superimposition of different target duration when plotted on a relative time scale—referred to as timescale invariance (Church, 2003; Almeida and Ledberg, 2010). As such, violations of the scalar property of interval timing may be considered a diagnostic test of the source of pathophysiological distortions in timing and temporal memory (Malapani et al., 1998; Meck, 2002b, 2005; Meck and Malapani, 2004; Cheng and Meck, 2007d; Buhusi et al., 2009).
Use of psychophysical timing procedures for isolating changes in clock speed and memory storage
Interval timing has been hypothesized to rely on an optimal level of dopaminergic activity in corticostriatal circuits modulated by serotonin and glutamate activity (Cheng et al., 2006, 2007a, b, c; Williamson et al., 2008; Coull et al., 2011). To date, a broad array of studies have shown that systemic injections of drugs that are believed to promote dopaminergic function (e.g. methamphetamine, cocaine and nicotine) produce horizontal leftward shifts in the psychophysical functions relating the probability of a response to signal duration [i.e. on perception tasks, a relatively shorter intermediate duration in a set has an increased tendency to be classified as ‘long’, and on performance tasks, a given duration tends to be under (re)produced (Meck, 1996; Coull, 2011)]. Psychopharmacological data from temporal bisection and peak-interval timing procedures typically utilize a ‘train-test’ paradigm. This paradigm serves as a powerful tool for studying the adjustment to the acute and chronic effects of a drug and its discontinuation (Meck, 1996). For example, rats trained on a bisection procedure following saline injections can be evaluated for the acute effects of methamphetamine on timing during randomly selected test sessions for which they are administered methamphetamine rather than saline. Under these conditions, methamphetamine typically produces an immediate, proportional leftward shift in the timing function(s) when responses controlled by time are separated from those responses that are not controlled by time (Meck, 1983; Cheng et al., 2007b). This leftward shift typically adjusts with continued training such that the drug and saline functions appear identical, although the drug continues to be administered. Dissociations can be demonstrated, however, when the drug administration prior to a session is discontinued and saline is administered instead. Under these conditions, an immediate rightward shift is observed of approximately the same magnitude as the original leftward shift. This horizontal shift also adjusts with continued training under saline and the subject's psychophysical functions return to normal. In contrast, subjects who acquire the initial temporal discrimination under the influence of the drug (e.g. methamphetamine or cocaine) may demonstrate effects on the rate of acquisition, but at steady-state performance do not appear any different from subjects that have acquired the discrimination following saline injections. When this chronic methamphetamine treatment is discontinued, however, an immediate rightward shift is observed that is proportional to the durations being timed (Meck, 1983, 1996). Taken together, these patterns of train/test drug administration under which subjects are either (i) trained under saline control conditions and later tested for the acute effects of the drug and its subsequent removal; or (ii) are trained under chronic drug treatment and are later tested for the acute effects of withdrawal, provide an unique opportunity for separating non-associative mechanisms of sensitization and tolerance from the associative mechanisms of learning and memory on timing and time perception (Buhusi and Meck, 2005; Williamson, 2008; Coull et al., 2011).
In a complementary fashion, a parallel series of studies have shown that systemic injections of drugs that are believed to inhibit dopaminergic function (e.g. cholecystokinin octapeptide, haloperidol, pimoside and raclopride) produce horizontal rightward shifts in psychophysical functions relating the probability of a response to signal duration [i.e. on perception tasks, intermediate longer durations in a set are less likely to be classified as ‘long’, and on performance tasks, a given duration tends to be over (re)produced (Meck, 1986, 1996, 2006a; MacDonald and Meck, 2004, 2005, 2006)]. This pattern of observed horizontal shifts is consistent with the idea that the speed of an internal clock is regulated by the ‘effective’ level of dopamine, with proportional leftward shifts indicative of an increase in clock speed (so the criterion amount of ‘clock ticks’ are accumulated faster) and proportional rightward shifts indicative of a decrease in clock speed [the required amount of ‘ticks’ are accumulated more slowly (Meck, 1996; Buhusi and Meck, 2002; Coull et al., 2011)]. In addition to speeding up or slowing down, subjective time can stop and cease to exist all together, such as when people experience feelings of being in the ‘zone’. In these instances, a person is highly focused on an internal context that is separate from the external reality. The exclusion of the extraneous world typically is not ‘all or none’, but is filtered according to the context. Yoga, Tai Chi and other forms of meditation seem to produce a similar experience in which subjective time ceases or stands still. Indeed, meditation uses the same technique of hyperfocusing on a single stimulus (mantra) while ignoring the outside world and ‘being at one with things’ is a typical description of the situation. A parallel situation seems to occur in crises or threatening situations that provoke fear and anxiety (Meck and MacDonald, 2007). It is not only targeted pharmacological manipulations to the clock stage of the Scalar Expectancy Theory system that can produce left- and rightward shifts in the timing function, other studies have shown a different pattern of horizontal shifts in timing functions following pharmacological-induced changes posited to affect the memory stage in Scalar Expectancy Theory. Such changes in temporal memory involve gradual rather than abrupt changes in the horizontal placement of timing functions. These gradual horizontal shifts are typically produced by cholinergic drugs (e.g. anticholinesterases such as physostigmine produce proportional leftward shifts, and acetylcholine receptor antagonists such as atropine produce proportional rightward shifts). The major feature of the horizontal shifts associated with changes in temporal memory is that they fail to re-normalize with continued training under the drug and only gradually normalize once the drug treatment is discontinued (Meck, 1983, 1996, 2002a, b, 2006c; Buhusi and Meck, 2005; Coull et al., 2011).
Populations that reveal pathophysiological differences in interval timing
We begin our review of pathophysiological differences with those clinical populations, e.g. patients with damage to the basal ganglia, who have known pathophysiological alterations in brain anatomy or neurochemistry localized in brain areas recently implicated in ‘normal’ human and animal studies of interval timing (for reviews, see Meck and Benson, 2002; Meck, 2005; Meck et al., 2008; Coull et al., 2011). Following from this, we review pathophysiological differences in timing and time perception in other disorders (e.g. autism, depression and schizophrenia) whose neurobiology is not so clear-cut.
Parkinson's disease
Parkinson's disease is characterized by atrophy of the substantia nigra and a depletion of dopamine releasing neurons that project to the caudate–putamen, and is therefore a widely studied model of basal ganglia dysfunction. Malapani et al. (1998, 2002) adapted a peak-interval timing procedure and manipulated whether patients were tested ON or OFF their levodopa medication (they were trained ON medication during which their timing is typically ‘normal’). These investigators found that when patients OFF medication were required to time a visual signal duration (e.g. 21 s), they tended to produce it as slightly longer, suggestive of a possible lengthening or ‘slowing’ of temporal processing (they showed scalar variance between their estimates ON and OFF medication). However, using a double-duration design (bi-peak procedure)—where the two durations are tested in separate trial blocks, but within the same session (e.g. 8 s and 21 s), patients with Parkinson's disease over-reproduced the 8-s duration and under-reproduced the 21 s duration to a considerable extent—a result referred to as the ‘migration effect’ because the peak times for the two target durations were drawn towards each other. Furthermore if the 8- and 21-s peak functions from the OFF medication condition are directly compared, they do not superimpose (which they usually do in the ON medication condition as well as for normal participants). Moreover, the ‘migration effect’ is apparently quite persistent and cannot be attenuated with corrective feedback and the relative extent of migration has sometimes been found to correlate with clinical akinesia, i.e. impairment of voluntary movement (Malapani and Rakitin, 2003). Collectively, the pre-ponderance of evidence from these experiments suggests the ‘migration effect’ results from a ‘coupling’ of the target durations in reference memory or changes in the dynamics and tuning of neural accumulators during the signal (Malapani et al., 1988; Malapani and Rakitin, 2003; Shea-Brown et al., 2006), although the observation that scalar variance was present between the ON and OFF conditions in the single-duration task, and that there was a significant source of non-scalar variance between the two durations in the OFF condition in the double-duration task, suggests that a deficit in attentional-set shifting might also be responsible for the ‘migration effect’ (Meck and Benson, 2002). Intriguingly, single duration differences and the ‘migration effect’ have not been obtained for millisecond durations, and may only be revealed when the magnitude of the durations tested exceeds 2 s (Koch et al., 2005, 2008a; see also Jones et al., 2008 for a discussion of the pharmacological basis of the ‘migration effect’).
Other related pathophysiological findings in patients with Parkinson's disease include reports that these individuals (verbally) under-estimate a given duration, and they over-reproduce various durations, even when they are encouraged to verbally ‘count’ seconds out loud (Pastor et al., 1992; Lange et al., 1995). That is, patients with Parkinson's disease were found to behaviourally produce 1 s as longer than 1 s in objective time and the extent of these pathophysiological differences (slowing of time) was correlated with severity of Parkinson's disease symptoms (Pastor et al., 1992). However, two of the findings we have just described are time performance measures—and as Parkinson's disease is characterized by motor impairments, it is particularly worthwhile to consider evidence from studies of time perception that are separated from motor performance.
In fact, Wearden et al. (2008) tested patients with Parkinson's disease (when they were both ON and OFF their medication) on a battery of perceptual timing tasks, including: temporal bisection, generalization, verbal estimation, threshold determination and a memory for duration task (essentially, the latter two procedures can be considered variants of an ordinality-comparison procedure). Patients OFF (and ON) medication did not demonstrate any significant pathophysiological differences in the location of the point of subjective equality in temporal bisection, produced ‘normal’ shaped generalization gradients and gave accurate verbal time estimations. In fact, pathophysiological differences were only obtained during ordinality-comparison arrangements (patients with Parkinson's disease showed reduced accuracy). However, the majority of these tasks employed subsecond stimuli (or those shorter than 2 s), and so it remains to be seen whether any pathophysiological differences in time perception to supra-second durations are revealed in patients with Parkinson's disease when tested OFF their medication. For instance, Smith et al. (2007) compared patients with Parkinson's disease tested ON medication to non-affected controls using visual and auditory durations in the supra-second range and found pathophysiological differences in the location of the point of subjective equality for temporal bisection and increased sources of non-scalar variance (difference limen and Weber ratio). Smith et al. (2007) accounted for their findings (particularly, increases in variability) by supposing a dopamine-related reduction in clock speed in patients with Parkinson's disease, as increases in clock speed are held to improve precision (Rammsayer and Classen, 1997; Rammsayer, 1999; Penney et al., 2005; Williamson 2008). Interestingly, Jones et al. (2008) reported significant impairments in temporal processing (e.g. violation of the scalar property) when patients with Parkinson's disease were tested either ON or OFF medication in comparison to healthy controls for time productions in the supra-seconds range (e.g. 30–120 s); suggesting that the timing deficits in patients with Parkinson's disease are pathophysiological, i.e. the effects of medication are secondary to the integrity of the basal ganglia, which is crucial for timing in both the subsecond and supra-second ranges (Jones and Jahanshahi 2009; Koch et al., 2009; Jahanshahi et al., 2010a, b; Harrington et al., 2011). These results also serve as an important reminder that the dose of dopaminergic medication given to patients with Parkinson's disease is typically titrated in order to obtain improvement in motor symptoms and not cognitive function. Hence some studies may show timing impairments in the ON medication condition due to under or over (more likely) medication (Pouthas and Perbal, 2004; Rakitin et al., 2006).
A number of functional MRI studies of time reproduction and time perception have shown no effect of dopamine replacement therapy on performance (Elsinger, et al., 2003; Jones and Jahanshahi 2009; Jahanshahi et al., 2010a, b; Harrington et al., 2011). Moreover, patterns of brain activation in a study of earlier stage participants did not support the ‘over-activation’ hypothesis mentioned above, at least in the context of time perception (Harrington et al., 2011). These authors also reported that dopaminergic medication does not restore striatal or cortical activation (Harrington et al., 2011). Nevertheless, compelling evidence has recently been reported that dopaminergic medication can increase structural corticostriatal connectivity between the caudate and the prefrontal cortex in temporal reproduction tasks, whereas the OFF medication condition ‘deactivates’ these circuits and leads to a greater reliance on the cerebellum for motor timing in patients with Parkinson's disease (Jones and Jahanshahi 2009; Jahanshahi et al., 2010a, b). In contrast, such enhanced coupling in the ON medication state has not been observed for temporal perception tasks (Harrington et al., 2011), suggesting the possibility of context dependency for these dopamine effects. The implications of this mixed pattern of results for the dopamine hypothesis of Striatal Beat Frequency Theory (this is described in the next section; but is essentially a neurobiological instantiation of the Scalar Expectancy Theory system) and for behavioural studies reporting no effect of medication therapy on temporal processing is unclear. One possibility is that while dopamine replacement improves motor symptoms, it does not adequately reinstate functioning in nigrostriatal and mesocortical systems that are vital for temporal processing (Meck, 2006b; Harrington et al., 2011). Due to the heterogeneity in Parkinson's disease, there is also likely considerable individual variability in the responsiveness of various neural networks to dopamine replacement therapy (Merchant et al., 2008a).
Although the interval-timing studies reviewed here studied patients with idiopathic Parkinson's disease at an intermediate disease stage with no clinical evidence of dementia, the interaction between disease stage and dopaminergic therapy in Parkinson's disease is not well understood in terms of effects on motor control, decision-making and interval timing (Rowe et al., 2008; Jones and Jahanshahi 2009; Jahanshahi et al., 2010a, b; Harrington et al., 2011). Nevertheless, the impairments in temporal cognition reported here (e.g. increases in variability, proportional rightward shifts when timing a single target duration and ‘migration effect’ when timing multiple target durations within the same session) occurs even in non-demented and early-stage patients with Parkinson's disease when tested OFF dopaminergic medication and are at least partially restored when tested ON medication (Malapani et al., 1998, 2002).
Schizophrenia
Schizophrenia is an acquired psychiatric disorder in which there is an apparent disintegration in the processes of thinking and emotional responsiveness, and the presence of auditory hallucinations, paranoia and delusions. It has therefore been characterized by some as a disorder of temporal coordination of information processing in the brain (Densen, 1977; Andreasen, 1999; Fuchs, 2007; Carroll et al., 2008; Lee et al., 2009). Schizophrenia has previously been associated with ‘distortions in time’, however, much like the study of autism, investigators are only just beginning to systematically investigate the ability of these individuals to estimate time using standardized psychophysical procedures. A recent study by Carroll et al. (2008), for example, employed a temporal bisection task using subsecond durations (300–600 ms), and both visual and auditory stimuli (Elvevag, 2003).
Carroll et al. (2008) observed that control participants and individuals with schizophrenia (the majority of whom were on antipsychotic medications—typical, atypical or both) demonstrated the standard ‘modality effect’ in that auditory signals were judged to be longer than visual signals of the same physical duration (Penney et al., 2000)—although the magnitude of the modality difference was reduced in the schizophrenia group. Surprisingly, however, patients with schizophrenia reliably produced ‘non-linear’ response classifications for visual durations with reversals in response classifications occurring near the midpoint of the signal range (i.e. geometric mean of the ‘short’ and ‘long’ anchor durations; in other words there is a ‘kink’ in the psychometric timing function). This result is similar to the temporal bisection ‘reversal effect’ reported for a pre-symptomatic rat model of Huntington's disease (Höhn et al., 2011) as well as for normal pigeons, mice and humans when duration discrimination in the temporal bisection task becomes progressively more difficult, i.e. as the ratio of ‘short’ to ‘long’ anchors durations became smaller (Penney et al., 2008). Taken together, these results suggest that individuals with schizophrenia given chronic antipsychotic medication (e.g. dopamine antagonists) have an increased difficulty in timing visual signals, perhaps due to a reduction in clock speed and/or increased variability in the timing of these signals. Moreover, the bisection of the auditory signals near the geometric mean of the ‘short’ and ‘long’ anchor durations suggests a disproportionate dominance of auditory clock readings in reference memory (at the expense of visual ones) given that the auditory bisection function is typically observed to be shifted to the left of the geometric mean and the visual bisection function to the right (Meck and Church, 1982; Penney et al., 1998, 2000; Lustig and Meck, 2001, 2011; Melgire et al., 2005; Cheng et al., 2011—but see Carroll et al., 2008). These rightward shifts and reductions in the sensitivity to time and feedback effects are similar to those reported for non-schizophrenic patients given chronic haloperidol (Lustig and Meck, 2005).
In a subsequent study, Carroll et al. (2009a) also administered a multi-second temporal bisection procedure with auditory signals to individuals with schizophrenia. They reported no differences in the location of the point of subjective equality in patients with schizophrenia (compared with unaffected controls). However, these individuals did reveal pathophysiological differences in the precision of their psychometric timing functions, as reflected by the difference limen and Weber ratio. The investigators supposed a common source of variance within the range of durations employed, ‘that is not specific to the timing of longer durations’ (Carroll et al., 2009a, p. 188). Computer modelling corroborated that there was more temporal variability in patients with schizophrenia. These temporal bisection results for auditory signals are similar to those reported by Lee et al. (2009) in that patients with schizophrenia underestimated duration (higher point of subjective equality) in a 400 versus 800 ms condition, but not in a 1000 versus 2000 ms condition even though temporal sensitivity was decreased for both conditions. The majority of patients with schizophrenia were again medicated in this study (as they are in most studies).
The fact that a wide variety of different antipsychotic drugs are taken by the patients with schizophrenia participating in the timing studies described above complicates the interpretation of the results. Although different antipsychotic drugs can be compared using their ‘chlorpromazine equivalents’ with the resulting dosages correlated with behavioural measures (Carroll et al., 2008), this does virtually nothing to control for the differential timing effects observed for typical (e.g. haloperidol) and atypical (e.g. clozapine) antipsychotics, as well as for the anticholinergic and anti-depressant drugs that patients with schizophrenia are frequently administered (e.g. Meck, 1996; MacDonald and Meck, 2005; Buhusi and Meck, 2007). As a consequence, Penney et al. (2005) tested healthy, unmedicated individuals deemed to be at ‘high (genetic) risk’ for acquiring schizophrenia (e.g. one parent diagnosed as schizophrenic). These authors reported a greater clock speed difference (between auditory and visual stimuli) for individuals at high risk for acquiring schizophrenia than is ‘normally ‘revealed by control participants, and implicated the cause as a ‘flicking switch’ that needs to be closed in order to transfer ‘pulses’ from the pacemaker to the accumulator. It was further supposed that individuals at high risk for acquiring schizophrenia had faster experience of duration in the auditory modality and a slower experience of duration in the visual modality that is processed less automatically than the auditory modality and requires greater attention to keep the switch closed—similar to the effect reported for patients with schizophrenia by Carroll et al. (2008)—see Penney et al. (1996, 2000). An assessment of the scalar property revealed that individuals at high risk for acquiring schizophrenia show superimposition of all psychometric functions, meaning that the difference in the timing of auditory and visual stimuli is multiplicative (rather than additive) further endorsing the ‘flickering switch’ interpretation (Penney, 2003; Penney and Tourret, 2005).
Davalos et al. (2011) recently conducted a functional MRI study comparing clinically stable patients with schizophrenia (neuroleptic naïve as well as those treated with traditional and non-traditional neuroleptics) to healthy control participants in an ordinal comparison timing task at two levels of difficulty. In the ‘easy’ condition, participants compared 70–300 ms tones with a 200 ms standard, whereas in the ‘difficult’ condition 160–240 ms tones were compared with a 200 ms standard. Although no significant group differences were observed in reaction times, the schizophrenia group made more ‘shorter’ and ‘longer’ categorization errors than controls at both levels of difficulty. Moreover, differential patterns of brain activation were observed for the two groups as a function of task difficulty. Overall, patients with schizophrenia exhibited lower levels of activation in neural circuits frequently associated with interval timing, including the supplementary motor area, insula/opercula and striatum (Coull et al., 2011). These group differences were observed to increase as a function of task difficulty and led the investigators to propose that failures of duration discrimination in patients with schizophrenia are consistent with a ‘general’ temporal processing deficit rather than being specific to sub- or supra-second time ranges (Davalos et al., 2003, 2011).
Autism
Idiopathic autism is a developmental spectrum disorder (meaning that there is a wide scale of severity of different symptoms within the population). Although a neurobehavioural disorder, its underlying pathophysiology is not well understood. However, ‘abnormalities’ in the function of the prefrontal cortex, basal ganglia and cerebellum have been heavily implicated (Bauman et al., 1997; Sears et al., 1999; Allen et al., 2004; Hollander et al, 2005; Haznedar et al, 2006; Voelbel et al., 2006) as have a variety of neurotransmitters, including dopamine and serotonin (Makkonen et al., 2008; Nakamura et al., 2010)—both of which have been implicated in timing and time perception (Coull et al., 2008, 2011; Sysoeva et al., 2010; Meck et al., 2011; Weiner et al., 2011).
Allman et al. (2011a) employed a temporal bisection task using two pairs of ‘anchor’ durations in different sessions (1 versus 4 s, and 2 versus 8 s). Children with autism were found to have point of subjective equality values located at a significantly shorter value (relative to unaffected controls) during testing with both pairs of durations. In fact for the lesser (in magnitude) duration pair (1 versus 4 s), the point of subjective equality was found to correlate with scores on diagnostic tests for language and communication, and working memory. A greater deviation in point of subjective equality was obtained with those participants who had the ‘worst’ communication and working memory function. There was also a significant group difference in Weber ratio for the greater (in magnitude) stimulus pair (i.e. autistic sensitivity was ‘worse’). Furthermore, the two timing functions from affected individuals superimposed to a lesser extent. Principled computer modelling of the obtained temporal bisection data revealed that individuals with autism appear to have a tendency to ‘truncate’ (or shorten) longer durations, and more variable time representations overall, particularly for longer durations. These results might also be interpreted by supposing that memories for both the ‘short’ and ‘long’ anchor durations influenced test responding to a different extent, as a function of the relative magnitude of the duration pairs (i.e. the ‘short’ anchor had a major influence during the 2 versus 8 s discrimination). Of course, the psychological explanation for why autistic individuals may adopt two different strategies to perform the temporal bisection task is unclear, but one possibility may be that they experience pathophysiological distortions in their ability to estimate longer durations per se (their data suggest those durations over 3.0–3.5 s). Allman et al. (2011a) also indicate that parents of children with autism tend to describe their child's sense of time as ‘poor’ [It's About Time questionnaire (Barkley et al., 1997); developed for children with ADHD].
In a study using temporal reproduction, Szelag et al. (2004) tested autistic individuals with either auditory or visual signals, and two different duration ranges (although these were both between 1.0–5.5 s). Across modality and duration set, individuals with autism tended to reproduce all durations ∼3.0–3.5 s. One possibility is that this pattern of results reflects an underlying deficit in flexible motor programmes (which is likely disordered in autism; Gidley et al., 2006), and this intriguing pattern has not been replicated in a more recent study. Martin et al. (2010) revealed that individuals with autism are less accurate in their temporal reproductions, and more variable, particularly at longer durations (which were under-reproduced).
In a separate study, children with autism were compared with non-affected controls on a specific range of durations (between 2 and 45 s) on three alternate forms of task: time estimation, reproduction and production (Wallace and Happé, 2008). These authors report no differences between autistic and unaffected individuals on any of these assessments. In fact, those individuals with autism were more accurate during reproduction (which is in contrast to the aforementioned pathophysiological findings). However, those affected with autism did reveal several interesting trends; they generally over-estimated the durations, and made under-productions (to a lesser extent; compared with controls). They also observed that the marked tendency to over-estimate duration tended to decrease (slightly) as the durations got longer, whereas time production performance was more consistent.
Interestingly, unaffected family members (parents or siblings) of individuals with autism demonstrate patterns of oculomotor timing behaviour indicative of abnormalities in left-lateralized corticostriatal circuits (Mosconi et al., 2010). These timing deficits may be a distinguishing feature of autism given that they have not been reported for other neuropsychiatric disorders and could be related to the atypical brain lateralization and language development associated with the disorder.
Attention-deficit hyperactivity disorder
The essential features of ADHD are inattention, hyperactivity and impulsivity. An extensive survey of timing-based findings from participants (usually children) with ADHD is presented by Toplak et al. (2006), and we will not repeat all these here (they do not all yield consistent results; and many are also related to timed motor programmes). It is usually found that on duration discrimination tasks (being able to perceive a noticeable difference between durations), that individual's with ADHD have higher difference thresholds, across the milliseconds to seconds range of stimulus duration, in both auditory and visual modalities. During temporal production and reproduction procedures, it is often (but not always) revealed that individuals with ADHD tend to underestimate durations, and their judgements have increased variability. In a recent study examining time estimation (verbal report), individuals with ADHD were found to produce less accurate judgements (more errors), which were also more variable (Pollak et al., 2009). Barkley et al. (1997) also reported that children with ADHD are rated more ‘poorly’ by their parents on questionnaires designed to evaluate their ‘sense of time, their referencing of time in their daily discourse with others, their ability to conform to directions containing time parameters, and their ability to meet deadlines associated with work assignments’ Barkley et al., 1997, p. 361). It remains to be determined whether the supra-second timing deficits presented by individuals with ADHD are best attributed to difficulties in timing per se or to a limited attentional capacity that impacts a variety of cognitive functions (Meck, 2005; Hwang et al., 2010).
Levin et al. (1996) examined the effect of nicotine (administered by transdermal patch) on time estimation in non-smoking adults with ADHD (tested OFF their normal medication, e.g. methylphenidate). Nicotine has been found to alleviate some of the attentional deficits associated with this disorder (as well as schizophrenia) due to its putative effect on dopamine release in striatum and prefrontal cortex (Cao et al., 2005). In the timing component of the study, nicotine was found to improve performance on 7 and 17-s peak-interval procedures, increasing both accuracy and precision by normalizing the proportional rightward distortions observed for the timing of visual stimuli OFF medication. These rightward horizontal shifts were attributed to deficits in attention (e.g. flickering of a mode switch that gates pulses from a pacemaker into an accumulator) and could be corrected either by feedback or nicotine administration—demonstrating synergy between behavioural and pharmacological interventions for ADHD (Levin et al., 1996, 1998; Meck, 2005; Groom et al., 2010).
What individual and pathophysiological differences reveal about ‘normal’ timing
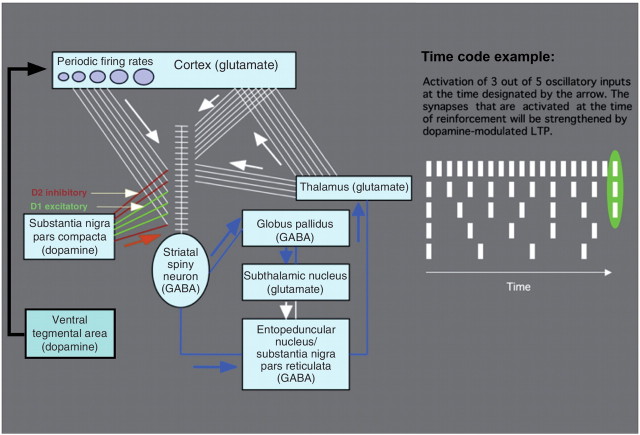
As already mentioned, we intend to discuss how some of the pathophysiological findings in time perception and timed performance described above and outlined in Table 1 might be understood within the ‘context’ of a recently described neurobiological model of interval timing. Briefly, in the striatal beat frequency model (Matell and Meck, 2000, 2004; Matell et al., 2003, 2011; Buhusi and Meck, 2005; Meck et al., 2008; Coull et al, 2011) duration estimation is based upon the coincidence detection of oscillatory processes in corticostriatal circuits as illustrated in Fig. 2. The striatal beat frequency model supposes that: at the onset of a to be timed signal, populations of cortical (and thalamic) neurons phase reset (and synchronize) and begin oscillating at their endogenous periodicities (the clock stage in Scalar Expectancy Theory). Dopamine release from the ventral tegmental area at the onset of the signal is believed to play a part in this resetting function for cortical neurons while also acting as a ‘start gun’, and dopamine release from the substantia nigra pars compacta at signal onset works in a similar fashion to reset the weights of the synaptic connections in the dorsal striatum (Matell and Meck, 2004; Buhusi and Meck, 2005; Jahanshahi et al., 2006; Meck et al., 2008). The detection of coincident activation of specific cortical oscillation patterns is the role of striatal medium spiny neurons (input cells of the basal ganglia). The adjustment of corticostriatal synaptic weights (akin to the memory stage in Scalar Expectancy Theory) allows the striatal spiny neurons to discriminate and become ‘tuned’ to specific patterns of coincident oscillatory activity, increasing their likelihood of firing upon similar patterns of cortical activation in the future. This property accounts for the close correspondence to aspects of interval timing and working memory performance, which are held to depend on the same neural representation of a specific stimulus (Lustig and Meck, 2005; Lustig et al., 2005). Given that oscillatory activation repeats itself at regular intervals (its period) and changes in a systematic manner as a function of time (its phase), these cortical oscillatory patterns can represent time intervals in the seconds-to-minutes range although their neural firing occurs in the milliseconds range. The striatal medium spiny neurons are able to detect these patterns, which are similar to musical cords, by acting as coincidence detectors or ‘perceptrons’ (Buonomano and Maass, 2009) and function much like the decision stage in the Scalar Expectancy Theory. Striatal output travels to the thalamus along two pathways: the direct (dopamine D1 receptor mediated) and indirect (dopamine D2 receptor mediated) (Graybiel, 2000; MacDonald and Meck, 2004; Buhusi and Meck, 2005), then loops back to the cortex and striatum, influencing the rate of oscillatory activity and permitting alterations in clock speed by changing the input to striatal spiny neurons (and producing a response). Differential activity in the direct and indirect pathways of the basal ganglia may serve to start, stop (pause) or reset the timing process (Matell and Meck, 2004, p. 152). Consequently, the striatal beat frequency model has the advantage of being consistent with the known neuroanatomy and neuropharmacology of interval timing while at the same time making testable predictions regarding the functioning of its components (Rao et al., 2001; Macar et al., 2002; Coull et al., 2004, 2008, 2011; Harrington et al., 2004a, b, 2010; Ivry and Spencer, 2004; Koch et al., 2005, 2008a, b, 2009; Jahanshahi et al., 2006; Stevens et al., 2007; Meck et al., 2008; Jones et al., 2009). A description of the quantitative neurophysiological parameters used in computer simulations of the striatal beat frequency model is provided in Appendix I.
Table 1.
Summary of individual differences and pathophysiological distortions in time perception and time performance
| Basic timing procedures | Individual differences | Neurological condition(s) |
|---|---|---|
| Bisection function (point of subjective equality ≈ geometric mean) equal influence of both ‘short and long’ anchors | Some ‘normal’ participant's exhibit greater influence of the ‘short’ anchor on the point of subjective equality | Autism may lead to greater influence of the ‘short’ anchor duration (Allman et al., 2011a). Left temporal lobe resection (in contrast to the right temporal lobe) produces over-estimation and depression produces underestimation of duration (Vidalaki et al., 1999; Melgire et al., 2005; Balci et al., 2009; Gil and Droit-Volet, 2009) |
| Auditory/visual differences in point of subjective equality when trained in same session—explained by ‘memory mixing’ | Some ‘normal’ participants do not exhibit the auditory/visual difference in point of subjective equality | Participants at ‘high risk’ for schizophrenia as well as individuals with schizophrenia exhibit greater auditory/visual—point of subjective equality difference—perhaps due to a relative decrease in attention and/or clock speed for visual signals. This is in contrast to participants at risk for affective disorders and those with temporal lobe resection (Melgire et al., 2005; Penney et al., 2005; Carroll et al., 2008) |
| Ordinal comparison procedure with multiple standards | Individual differences in ‘memory mixing’, i.e. some ‘normal’ participant's display little or no ‘mixing’ of the different standards in memory | ‘Memory mixing’ effect can be influenced by feedback with differential effects of valence (e.g. positive versus negative feedback effects)—suggesting the involvement of dopamine (Gu and Meck, 2011b) |
| Peak-interval timing procedures with associated measures of accuracy (peak time) and precision (peak spread) | Individual differences in accuracy and precision (Rakitin et al., 1998; Meck, 2002a, b). Individual differences in ‘migration’ with multiple standard durations (Malapani et al., 1998) | Individual differences in peak time in ADHD and normal adults as a function of drug treatment (nicotine or haloperidol) and the probability of intertrial interval feedback. Dopamine-controlled regulation of clock speed is used to explain the drug and feedback effects (Levin et al., 1996; Lustig and Meck, 2005; Meck, 2005). Patients with Parkinson's disease tested OFF their levodopa medication exhibit large ‘migration’ effects—suggesting a role for dopamine in this form of ‘memory mixing’ (Malapani et al., 1998; Koch et al., 2005, 2008a) |
| Ambiguous tempo judgement paradigms | Large individual differences in the strength of beat based versus interval timing are observed (Grahn and McAuley, 2009) | Quinpirole (dopamine D2 receptor agonist) sensitized rats more readily engage in rhythmical (beat-based) timing behaviour reminiscent of the ‘non-functional’ fixation to time observed in obsessive–compulsive disorder (Gu et al., 2008, 2011a) |
| Time estimation up to 60 s | Individual differences in time perception due to spatial asymmetries and ‘normal’ levels of neglect in healthy individuals (Vicario et al., 2008; Grondin, 2010; Hurwitz and Danckert, 2011) | Underestimation of time in patients with unilateral neglect (Danckert et al., 2007) |
Figure 2.
Striatal beat frequency model of interval timing. In this model, intervals are timed via striatal spiny neurons that monitor activation patterns of oscillatory neurons in the cortex. These cortical neurons have patterns of activity that fire with different frequencies and converge onto spiny neurons, as illustrated. At the beginning of an interval, these oscillating neurons are synchronized and the status level of the spiny neurons reset by phasic dopaminergic input from the ventral tegmental area and substantia nigra pars compacta, respectively. The delivery of reinforcement at the target duration produces a pulse of dopamine thereby strengthening the synapses in the striatum that are activated as a result of the beat frequency pattern of these cortical neurons at that specific point in time. In this manner, mechanisms of long-term potentiation (LTP) and long-term depression are used to strengthen and weaken synaptic weights in order to produce a record in memory of the target duration. Later, when the same signal duration is timed again, neostriatal GABAergic spiny neurons compare the current pattern of activation of these cortical neurons with the pattern stored in memory in order to determine when the target duration has been reached. When the clock and memory patterns match as determined by coincidence detection, the spiny neurons fire to indicate that the interval has elapsed. In this model, clock speed is determined by the levels of tonic dopamine–glutamate activity in ventral tegmental area–cortical pathways, which modulates the frequency of cortical oscillations (Cheng et al., 2006, 2007a, b, c). Adapted from Matell and Meck (2004).
As described above, the role of the basal ganglia in temporal processing has been typically investigated in patients with Parkinson's disease, albeit with mixed results (Perbal et al., 2005; Smith et al., 2007; Jones et al., 2008; Koch et al., 2008a; Merchant et al., 2008a; Wearden et al., 2008). Studies involving patients with Parkinson's disease also suggest difficulties in beat extraction and the comparison of rhythmic sequences (Grahn and Brett, 2009; Grahn and McAuley, 2009). However, Parkinson's disease is a progressive neurodegenerative disease, and besides medication and different Parkinson's disease subgroups, some of the heterogeneity of the respective results may be due to the variable extent of cortical damage in this population, which can be minimal or absent in patients with basal ganglia lesions. As a consequence, the observation of timing deficits in patients with focal basal ganglia lesions provides strong evidence for the involvement of the caudate and putamen in timing and time perception (Schwartze et al., 2011; but see Aparicio et al., 2005; Coslett et al., 2010 for possible exceptions). Moreover, mesolimbic, nigrostriatal and mesocortical phasic dopamine release are crucial to the striatal beat frequency model of temporal processing, e.g. problems in timing and time perception could arise in the synchronization of cortical oscillations at signal onset (ventral tegmental area to cortical dopamine burst), or resetting of striatal spiny neuron membrane potentials (substantia nigra pars compacta to striatum dopamine burst), or tonic dopamine levels regulating clock speed during a trial as described by MacDonald and Meck (2004, 2005), Matell and Meck (2000, 2004) and Meck (2006a, b).
Parkinson's disease
The ‘migration effect’ observed in the double-duration condition (Malapani et al., 1998) cannot be easily accounted for by the standard type of information processing model that Scalar Expectancy Theory provides (Meck and Benson, 2002; Shea-Brown et al., 2006). Within the context of the striatal beat frequency model, however, it may be accounted for by assuming that this dopaminergic effect arises from a decrease in the rate of cortical oscillations and the coupling of striatal spiny neurons as a result of reduced dopaminergic input. Such effects have been observed as a result of dopamine cell loss in the ventral tegmental area and substantia nigra pars compacta, respectively (Matell and Meck, 2004; Meck, 2006a, b). If spiny neurons representing different target duration (e.g. 8 and 21 s) were to couple and form cell assemblies in response to dopamine depletion, then ‘migration’ of the durations that they represent would be expected (Matell and Meck, 2004; Humphries et al., 2009).
As described previously, a recent functional MRI study using an ‘ordinality comparison’ procedure (Harrington et al., 2011) has indicated that patients with Parkinson's disease, both ON and OFF levodopa medication, reveal disturbances in striatal activation during the timing of both the standard and comparison durations (task difficulty was deliberately adjusted so that any differences in behavioural timing functions would be attenuated, which are considered by some to introduce potential confounds to neural interpretation). Harrington et al. (2011) reported Parkinson's disease differences across a distributed ‘normal’ timing system (for a review, see Macar and Vidal, 2009) and report Parkinson's disease timing dysfunction in multi-sensory association areas. According to the striatal beat frequency model, striatal activity subserves a ‘central clock’—hence the observed Parkinson's disease dysfunction for both durations in a sequence pair. Moreover, striatal beat frequency predicts that striatal activation would be different across modalities if separable sensory processes promote different corticostriatal interactions. According to the striatal beat frequency model, delayed and depressed striatal activation early in the course of interval encoding should reflect reduced sensitivity in detection and/or integration of cortical oscillatory states, thereby producing the observed increases in timing variability for patients with Parkinson's disease tested both ON and OFF dopaminergic medication (Harrington et al., 2011).
Schizophrenia
The presented evidence suggests that individuals at high risk for acquiring schizophrenia exhibit exaggerated differences in clock speed between auditory and visual modalities with the auditory modality dominating timing and time perception in terms of increased clock speed and the sampling of memory distributions for anchor durations. On the other hand, chronically medicated patients with schizophrenia display more muted differences in the experience of time ‘between’ sensory modalities. That is, they do not appear to judge an auditory stimulus (of a given duration) as longer than a visual stimulus to the same extent as control participants. This ‘modality effect’ is presumably a critical component of functional intersensory integration across different modalities (i.e. the sight and sounds of a person's speech). It is easy to imagine how differences in the cortical time base for sensory modalities might create problems of coincident detection within the striatal beat frequency model—furthermore, there is a recent hypothesis of schizophrenia that attributes asynchronous cortical network oscillations to impairments in cognitive function (Gonzalez-Burgos and Lewis, 2008). For instance, distorted cortical activation and impairment in sensory integration ostensibly suggests that signal durations might be poorly accumulated. Conceptually, one might even conceive of ‘echoes’ of interference in the process of estimating time (hallucinations), as fragments of time estimations from different modalities may not be combined in an appropriate fashion. This might even be assumed to produce failures in the adequate perception of temporal order—particularly action or agency; see also Carroll et al. (2009b) for further pathophysiological differences during the continuation phase of a finger-tapping task in affected individuals with schizophrenia and Stavitsky et al. (2008) for the occurrence of hallucinations in Parkinson's disease as a function of related temporal factors.
Given the observation that modality effects are exaggerated in individuals at high risk for acquiring schizophrenia, it would be important to determine whether these auditory–visual differences vary systematically as a function of the expression of schizophrenia symptoms and antipsychotic drug treatment. Our expectation is that these modality effects would be greatest in first episode, antipsychotic drug naïve patients with schizophrenia given the potential for long-term antipsychotic drug treatment to reduce differences in cortical and basal ganglia volumes between patients with schizophrenia and controls (Glenthoj et al., 2007; Ebdrup et al., 2010; Gutiérrez-Galve et al., 2010). The most straightforward assumption would be that individuals at high risk for acquiring schizophrenia lie on a continuum somewhere between medicated and drug naïve schizophrenia patients. In this fashion, the striatal beat frequency theory could account for the different patterns of modality effects in individuals at high risk for acquiring schizophrenia and patients with schizophrenia if basal ganglia volumes were found to co-vary as a function of the expression of schizophrenia symptoms and duration of antipsychotic drug treatment, taking into account potential differences between typical and atypical antipsychotic drugs (Scherk and Falkai, 2006; Glenthoj et al., 2007).
Autism
The striatal beat frequency model allows us to speculate on certain characteristic aspects of ‘autistic’ behaviours. For instance, there is a close correspondence between the neurobiology of the model's proposed timing circuits and those that underlie certain stereotypic behaviours. For example, dopamine D2 receptor antagonists (e.g. haloperidol) separately decrease stereotypy and produce a rightward shift (over-estimation) in timing functions (Lustig and Meck, 2005). The dopamine hypothesis of autism arose partly from observations that haloperidol is effective in alleviating certain aspects of the autistic behavioural phenotype (Volkmar and Pauls, 2003), which indirectly might imply an increase in clock speed in autism that is normalized by medication. However, across the studies we report, there are few indices that would reveal such mediation effects (but see Wallace and Happé, 2008). Consistent with altered levels of dopamine in autism, it might be conjectured that these individuals possess an aberrant cortical resetting function, which in turn may serve to potentiate cortical asynchronizations (in fact, a failure to resynchronize the oscillations is perhaps the most fundamental deficit with respect to its potential impact on duration estimation; as in schizophrenia). There is reasonable evidence of disordered cortical synchronization and ‘abnormal’ temporal binding of stimulus input in autism (e.g. Brock et al., 2002; Rippon et al., 2006); in fact, these individuals have been found to ‘bind’ sensations over an extended window of stimulus onset asynchronies than is typical, which is probably related to pathophysiological alterations in multisensory function (Foss-Feig et al., 2010). This type of temporal account resonates strikingly well with the weak central coherence hypothesis of autism—the idea that a limited ability to understand context or to ‘see the big picture’—underlies the central disturbance in autism and related autism spectrum disorders (Minshew et al., 1997). However, a word of caution is that the ‘mechanics’ of an interaction between temporal integration in timing and other cognitive processes is currently uncertain (see Meck and Benson, 2002, p. 207 for a fuller review). As we suggested in the context of findings in schizophrenia, it seems reasonable to suppose that disturbed coincident detection of (aberrant) oscillatory states could interfere with the starting (or ‘resetting’) of the interval clock (integrated by the striatum), and increase timing variability (which was found at a general level by most hitherto reported findings in autistic disorder).
Attention-deficit hyperactivity disorder
An intriguing example of the neuropharmacological basis of interval timing is illustrated by the results obtained from college students diagnosed with ADHD given methylphenidate maintenance therapy (Meck, 2005). During a discrete-trials peak-interval procedure with 7 s and 17 s target durations, a blue square transitions to magenta at the appropriate target duration during training trials, and thereafter, participants are requested to reproduce the target duration signalled by the duration of the blue square for a sequence of test trials after which a distribution of their responses is plotted on a relative timescale at the completion of the trial during the intertrial interval. This intertrial interval feedback is displayed on the computer monitor and provides the participant with information concerning the relative accuracy and precision of their timing behaviour on the just completed trial. Intertrial interval feedback can be randomly presented following a fixed proportion of trials (in this case 25 and 100%). As reported by Meck (2005), when the participant is provided with intertrial interval feedback on 100% of the trials the peak-interval functions are centred at the correct target durations showing excellent accuracy of the reproduced intervals. In contrast, when intertrial interval feedback is provided on only 25% of the trials, a proportional rightward shift is observed in the timing of the 7 s and 17 s intervals, reflecting a discrepancy in the accuracy of temporal reproductions that is not observed in normal participants (Rakitin et al., 1998; Lustig and Meck, 2005). This rightward shift is accompanied by a broadening of the peak-interval functions indicating a decrease in temporal precision with lower levels of feedback. Both of these findings are consistent with a slowing of the internal clock as a function of the probability of feedback and may be the result of a deficit in attention mediated by the flickering of a switch that gates pulses from a pacemaker into an accumulator or by a reduction in cortical oscillation frequencies (Penney et al., 1998, 2000; Meck and Benson, 2002; Lustig, 2003; Penney, 2003; Lustig and Meck, 2005; Coull et al., 2011). Interestingly, when participants are given a stimulant drug (e.g. 7 mg/day transdermal nicotine skin patch) that increases dopamine levels, the effects of 25% intertrial interval feedback are enhanced and produce levels of temporal accuracy and precision that are equivalent to the 100% intertrial interval feedback condition in both the medicated and unmedicated states. These results suggest an equivalence of the intertrial interval feedback effects and the types of pharmacological stimulation provided to patients with ADHD by drugs such as nicotine and methylphenidate (Levin et al., 1996, 1998). These findings also support the proposal that deficits in attention can lead to the underestimation of signal durations in a manner consistent with a slowing of an internal clock that is sensitive to dopaminergic manipulations whether they are produced by behavioural (intertrial interval feedback in a video game format) or pharmacological (nicotine or methylphenidate) therapeutic treatments (Koepp et al., 1998; Buhusi and Meck, 2002, 2005).
What pathophysiological differences reveal about the psychology of the clinical phenotypes
Parkinson's disease
The disruption of the ‘temporal dynamics’ of neural activation and the slowing down of processes involved in timing and time perception as a result of Parkinson's disease and its associated depletion of dopaminergic function is likely to impact numerous cognitive functions, including attention and memory (Harrington et al., 1998, 2010; Meck and Benson, 2002; Perbal et al., 2005; Jahanshahi et al., 2010a, b). Processing speed, for example, has been used as a measure of cognitive efficiency and/or cognitive proficiency as related to higher order function (Mega and Cummings, 1994; Benke et al., 2003). As a consequence, the potential links between temporal processing and other classic features of Parkinson's disease (e.g. planning, executive processing and cognitive inflexibility) are likely to become a major focus of future studies. In particular, recent functional MRI findings have shown disturbances in cortical systems besides ‘sensorimotor’ areas, including systems associated with working memory and memory encoding and retrieval (Harrington et al., 2011). These findings indicate that mesocortical dopamine pathways, in addition to the nigrostriatal dopamine pathways that degenerate in Parkinson's disease, contribute to temporal processing distortions while also providing support for a ‘disconnection syndrome’ view of Parkinson's disease in which coordination among subcortical and cortical structures is critical to cognition, emotion, perception and motor function (Cronin-Golomb, 2010).
Schizophrenia
Any distortions in the ability to estimate time as we have discussed might be presumed to further perpetuate temporal distortion. Posited differences in time estimation have a historical basis in the analysis of schizophrenia, and they too have been discussed within the concept of the ‘specious present’. Borst and Cohen (1987) surmised: ‘according to Fraisse (1984), a duration of up to about 3 s is perceived as a quantity whose beginning has not yet been stored in memory while in longer durations memory intervenes in the making of a global judgement about the duration. On the basis of this distinction, one could implicate a specific deficit in time estimation’ in schizophrenia (Borst and Cohen, 1987, p. 332). This gives some credence to the opinion that the scope of this platform (in terms of the integrity of the timing signal, and its capacity for multisensory integration) is related to aspects of what has become known as the ‘specious present’ which is, in some studies of other populations, assumed to the basis by which we can think forward and backward in time, and which forms the scaffolding for much of our ‘mental lives’.
Autism
Certain pathophysiological differences in individuals with autism were notable with respect to the emphasis that was focused in the duration range of 3.5–5.0 s (Szelag et al., 2004; Allman et al., 2011a), which may (or may not) be of some significance to our understanding of autistic disorder. Szelag et al. (2004) claimed that the processing of stimulus duration was disconnected from the temporal platform of the ‘specious present’, widely assumed to be between 3.0 and 5.0 s (Michon, 1978), but the findings of Allman et al. (2011a) reveal such difficulties only for the experience of longer durations, which are presumed to exceed the temporal limit of this processing ‘boundary’. This in turn, suggests autistic individuals may experience problems linking successive periods or ‘platforms’ together (Poppel, 1997, 2009), hence they may appear relatively insensitive to longer durations. This hypothesis is consistent with theories describing autism in terms of ‘weak central coherence’ (Happé, 1999; Nakano et al., 2010). It has also been previously suggested that sensory deprivation and isolation are accompanied by the subjective shortening of duration (Block, 1979, 1990), and it is an interesting possibility that the characteristic aloofness of autistic disorder (and lack of social engagement) might be somehow related.
It also seems likely that the ability to estimate duration is inextricably linked to being able to ‘think forwards in time‘, which has itself been suggested to approximate the relative length and complexity of an autistic child's restricted or repetitive behavioural and cognitive repertoire (Boucher, 2001; Allman and DeLeon, 2009). Perhaps one of the most intriguing pathophysiological findings in individuals with autistic disorder is the revelation that they experience problems with ‘diachronic (Montangero et al., 1996), that is: they are less likely to think about past or future stages of a current situation; have difficulty understanding that things can change or evolve over time but remain the same thing; and understanding that successive states or events are part of a unitary whole (Boucher et al., 2007). They also reveal selective difficulties on tasks that require a form of temporal dimension, such as thinking about the past (episodic memory; Maister and Plaisted, 2009) and the future (planning; Ozonoff et al., 1991)—that are considered by researchers of animal cognition to be aspects of ‘mental time travel’ (Clayton et al., 2003). It may finally be of interest to note that the 24-h circadian rhythm and more specifically levels of melatonin appear abnormal in autism (Nir et al., 1995). That is, there may be grounds to suppose pathophysiological deficits in timing across a wide range of scales that might be affected by the product of some ‘core’ aspect of an individual's inability to adequately estimate duration.
Attention-deficit hyperactivity disorder
Impulsivity displayed in ADHD and other disorders is, at least in part, held to be dependent on a (dopamine-mediated) propensity to choose smaller sooner over larger later rewards (in addition to other decision-making functions; such as a lack of inhibition of prepotent motor responses, over-weighting of rewards relative to losses and a failure to slow down in the face of decision conflict). This requires that both the magnitude of the reward and its relative distance in time, contribute to the assignment of ‘value’ (choice should be directed towards the reward with highest subjective ‘value’). It might be suggested that in addition to being mediated by reward learning (the dominant approach), such decision-making processes require some form of internal temporal scale [in ‘normal’ humans at least (Critchfield and Kollins, 2001)]. For the sake of parsimony, let us suppose that the ability to estimate duration (interval timing) might be related to (or form part of the basis of) an internal temporal framework (dimension) that is recruited to make such decisions (i.e. one might be some proportional transform of the other); in fact, both ‘time-keeping’ processes have been linked to the striatum [in striatal beat frequency and in temporal discount rate (Buhusi and Meck, 2005; Wittmann et al., 2007a, b; Wittmann and Paulus, 2008)]. Under these assumptions, pathophysiological increases in clock speed might be supposed to distort the breadth of an ‘imagined’ time frame, such that it (an expected ‘hypothetical’ delay) may be perceived as ‘too long’. It has been revealed (in ‘normal’ adults) that excess dopamine levels (by the administration of levodopa versus placebo) can selectively increase choice towards more immediate rewards, by influencing the rate of the discounting function, such that increases in delay appear to become intolerable (Pine et al., 2010). In fact, some patients with Parkinson's disease taking levodopa/carbidopa medication experience a variety of behavioural side effects (such as increased impulsivity, hypersexuality and pathological gambling). That clinically ‘impulsive’ populations [i.e. ADHD and drug addicts; who may also reveal clock speed deficits (Barkley et al., 1997; Wittmann et al., 2007a)] have also been found to produce characteristic differences in psychometric discounting functions (Bickel and Marsch, 2001) lends some support to the idea that underlying interval-timing processes might be directly related to distortions of temporal evaluations affecting choice and impulsive behaviours in certain individuals.
Conclusions
The experience of time is presumably of fundamental importance for how we make sense of the world. In fact, difficulties ‘making sense of the world’ may characterize certain clinical neurobehavioural or psychiatric disorders of cognition (e.g. schizophrenia and autism), although in today's medical profession, there is no disorder solely characterized as a disorder of timing. However, certain pathophysiological differences have been reported, particularly, in individuals affected with Parkinson's disease, schizophrenia, autism or ADHD. Moreover, such pathophysiological distortions may be particularly amenable to analysis according to a recently developed neurobiological model of interval timing (striatal beat frequency model; Matell and Meck, 2004), couched within a traditional information processing conceptual framework (Scalar Expectancy Theory; Gibbon et al., 1984). Collectively, these pathophysiological findings suggest that the estimation of duration is a fundamental ‘basic unit of ability’ on which other cognitive and behavioural processes are based. It might also be conjectured that the integrity of this ability is related to observable sensations and behaviours (such as poor intersensory integration and stereotypy), and even to an individual's internal ‘timeline for life events’. In fact, certain clinical populations display differentially shaped timing functions that are characteristic of their specific classification, thus, for example, allowing for the dissociation of various affective disorders [e.g. depression and mania (Sévigny et al., 2003; Bschor et al., 2004; Gil and Droit-Volet, 2009)]. The burgeoning interest in time perception and questions as to how it might be disturbed in certain clinical populations will undoubtedly hasten our understanding of timing-related disorders, but also, might hopefully go some way to remediating certain symptoms. For instance, aside from potential pharmacological manipulations designed to affect clock speed (Meck, 2005), the advancement and implementation of therapeutic/educational external supports designed to facilitate the temporal dimensions of emotion and cognition might be particularly effective in promoting adaptive behaviour (Lalli et al., 1994; Droit-Volet and Meck, 2007; Grondin, 2010). In fact, difficulties with time management skills are a pervasive problem in a variety of disorders of adaptive psychological function (i.e. neurological patients), and picture schedules, timers or timelines for upcoming events in time are often very effective in ameliorating difficulties. Recent reports, for example, have suggested that computer-based rhythm and timing training in children with ADHD contributes to improvements in attentional focus and time tracking (Leisman and Melillo, 2010; Leisman et al., 2010), whereas training-induced coordination and coherence of oscillation frequencies within thalamocortical pathways between hemispheres may support cognitive and motor improvement in autistic spectrum disorder (Melillo and Leisman, 2009). Moreover, improvements in gait speed, stride length and depression indices associated with Parkinson's disease have been observed following the rhythmic auditory stimulation provided by music therapy (Paccehetti et al., 2000; Hayashi et al., 2006). The beneficial effects of these different forms of rhythmical entrainment suggest the obligatory nature of synchronized oscillatory activity in targeted corticostriatal regions subserving interval timing (Gu et al., 2011a).
As previously noted, the major focus of our review has been on the cortex and basal ganglia—including the dorsal striatum, which receives its primary inputs from motor sensory, premotor and dorsal prefrontal cortices, and its interaction with the ventral striatum, which receives afferent inputs from orbitofrontal cortex, amygdala, hippocampus and anterior cingulate (Buhusi and Meck, 2005; Meck, 2006a, b; Meck et al., 2008; Coull et al., 2011). It is important to note that the cerebellum is another subcortical structure that has been proposed to be involved in precise timing and is impacted in numerous ways by most of the pathophysiological conditions discussed in this review (Ivry, 1996; Casini and Ivry, 1999; Diedrichsen et al., 2003; Spencer et al., 2003; Spencer and Ivry, 2005; Koch et al., 2007; Yu et al., 2007; Andreasen and Pierson, 2008; Picard et al., 2008; Gillig and Sanders, 2010; Gooch et al., 2010). The parallel here is that both the basal ganglia and cerebellum are central components of reciprocal neural circuits connecting with specific cortical regions as illustrated in Fig. 3 (Middleton and Strick, 1997). Moreover, an integrative model of timing and time perception has been recently proposed in which two parallel systems are required in order to account for the full range of durations resolved by the ‘internal clock’ (Madison, 2001; Santamaria, 2002; Ivry and Schlerf, 2008). This model includes a bottom-up system for timing in the milliseconds range—considered important for motor coordination and computed by the cerebellum. The other component involves a top–down system for timing in the seconds-to-minutes range—considered important for temporal estimation and computed by frontal–striatal circuits that are able to concatenate smaller intervals generated locally or by the cerebellum. To evaluate this model, Santamaria (2002) simulated a neural network of cerebellar activity that represented the millisecond timing system. Contrary to the initial predictions of non-linearity in the output of this network, simulations indicated that timing error increased linearly as a function of interval length, but drift in the variability of the model's output showed a systematic non-linearity (Crystal, 2003). It was also predicted that transfer of timing between different effector systems (e.g. left and right hands) would be minimal due to motor-specific interval learning by the cerebellum. In contrast, model simulations provided evidence for transfer of timing between hands indicating an unexpected robustness of the frontal networks and a surprising degree of independence from the cerebellum. One conclusion that can be drawn from this type of analysis is that frontal–striatal circuits are apparently able to rescale durations in a proportional manner and compensate for error differences generated by the cerebellum. This type of ‘scalar’ representation of intervals (Gibbon et al., 1984; Gibbon and Church, 1990) may contribute to the observed transfer among different effector systems by allowing for the encoding of event durations in a less motor-specific fashion and establishes a manner in which different timing systems (e.g. cerebellum and basal ganglia) can interact across a wider range of durations (e.g. milliseconds to hours). Such an analysis also allows for the identification of transitions from predictive to reactive modes of temporal processing following pathophysiological changes in a variety of cortical and subcortical structures involved in timing and time perception (Ghajar and Ivry, 2008; Coull et al., 2011; Harrington et al., 2011).
Figure 3.
A diagram of the corticostriatal and corticocerebellar circuits proposed to be involved in the interval-timing and motor-control components of procedural learning that are dysfunctional in Parkinson's disease and schizophrenia (Pascual-Leone et al., 1993; Andreasen et al., 1999; Kumari et al., 2002; Doyon et al., 2003). Full coloured lines represent excitatory input to various areas. Dashed lines and black lines represent inhibitory input to areas. Adapted from Meck (2005). AV = Anterior ventral; GPe = globus pallidus external capsule; GPi = globus pallidus internal capsule; IO = inferior olive; MTL = medial temporal lobe; PN = pontine nuclei; Red N = red nucleus; Ret N = reticular nucleus; SNc = substantia nigra pars compacta; VA = Ventral anterior; VLc/x = ventrolateral thalamic nucleus—caudal and area × divisions; VLm = ventrolateral medial thalamic nucleus; VLo = ventrolateral thalamic nucleus—oral division; VN = vestibular nuclei.
Funding
This work was supported in part by a Eunice Kennedy Shriver National Institute of Child Health & Human Development career development award (Pathway to Independence) (K99 HD058698 to M.J.A.).
Glossary
Abbreviations
- ADHD
attention-deficit hyperactivity disorder
Appendix I
Striatal beat frequency model simulation parameters based on Matell and Meck (2004).
The corticothalamic oscillation period was specified as having a mean of 10 ± 1.6 Hz. This is derived from a normal distribution of neuronal oscillation periods spanning a range of 5–15 Hz that is typical for corticostriatal neural activity.
Oscillation variability within trials was set so that 1 SD was equivalent to 1% of the input's period, normally distributed.
Oscillation variability between trials was defined to be Gaussian with a mean of 0 and a SD of 5% of the oscillation period.
The integration period for coincidence detection by striatal spiny neurons of oscillating corticothalamic inputs was set at 25 ms in order to conform to the electrophysiological data reporting this as the average membrane time constant for spiny neurons.
The striatal spiny neuron firing threshold was placed slightly below the level at which peaks at the quarter points would induce spiking, i.e. at 50% of the maximal neural activity that occurred at the time of reinforcement.
Firing threshold variance for striatal spiny neurons within trials needs to be specified because striatal membrane properties are dynamic, showing slowly inactivating and slowly recovering potassium currents, which effectively lowers and raises the threshold for maintenance of the depolarization state by ∼30%. Consequently, in order to conform with the electrophysiological data, the simulated within-trial firing threshold decreased by 30% over the first second in a linear manner following the first crossing, and then increasing symmetrically after each subsequent threshold crossing while being reset to the lower threshold following each crossing.
In order to conform to the electrophysiological data, the firing threshold was varied between trials in a Gaussian manner with a mean of 0 and a SD of 4% of the selected threshold.
Although striatal medium spiny neurons are considered to have up to 30 000 inputs from cortical and thalamic neurons, we used 15 000 inputs in order to simplify the simulations while still maintaining a reasonable degree of physiological accuracy. Each input was weighted at +1, if it was activated with the previous 25 ms at the time of reinforcement and 0 otherwise. These weights were used for simplicity and are intended to accurately represent the continuous strengthening and weakening of input due to the long-term potentiation or long-term depression expected during conditioning (training).
In many models, parameters are free to vary so as to best fit the data. In the reported simulations of the striatal beat frequency model, only the threshold estimate and oscillation variability were allowed to vary in order to fit the experimental data (Matell and Meck, 2004). Estimates of all other parameters were based on neurobiological data or were fixed once a reasonable outcome was achieved for baseline conditions (e.g. oscillation speed) prior to drug treatments. As such, this relatively long list of parameters reflects functional components of the striatal beat frequency model more than it reflects a wide range of free parameters to be adjusted for fitting purposes. It should also be noted that this coincidence-detection model would work equally well for alternative forms of the time base. For example, if clusters of cortical neurons all fired with the same underlying membrane potential/oscillation period, the summed output of these neurons would be sinusoidal. Consequently, this sinusoidal activity would be the primary input to the striatal spiny neurons (as opposed to spike activity only at the peak of every oscillation), and the mechanism for pattern detection would involve a type of fast Fourier transform (see Matell and Meck, 2004 for additional details). Certain aspects of the striatal beat frequency model are similar to the state-dependent network model proposed by Karmarkar and Buonomano (2007). Intrinsic timing models of this sort assume that timing is an inherent property of neural processing and that ‘the duration of a stimulus is coded by the same neural elements that respond to other sensory properties of that stimulus’ (Spencer et al., 2009, p. 1854). In this sense, the same cortical oscillatory mechanisms that code auditory and visual stimuli also converge on striatal medium spiny neurons that can detect patterns or states of neural firing and the sequential transitions among these states. In this sense, the timekeeper of the striatal beat frequency model is not a ‘dedicated’ clock in that it makes use of neural processes that are coding other aspects of the stimulus, including basic working memory processes (Lustig et al., 2005). On the other hand, the timing functions of these distributed circuits can be ‘isolated’ and studied as if they function as the core of a ‘dedicated’ timer because its output demonstrates ‘time scale invariance’ and exhibits consistent properties across a relatively wide range of durations from milliseconds to minutes (Buhusi and Meck, 2005; Meck et al., 2008; Coull et al., 2011)—something that the proposed state-dependent network models (which are intrinsic to the cortex) are currently unable to offer (Buonomano, 2007; Karmarkar and Buonomano, 2007; Spencer et al., 2009).
References
- Allan L. The perception of time. Percept Psychophys. 1979;26:340–54. [Google Scholar]
- Allan L. The influence of scalar timing model on human timing research. Behav Process. 1998;44:101–17. doi: 10.1016/s0376-6357(98)00043-6. [DOI] [PubMed] [Google Scholar]
- Allan L, Gibbon J. Human bisection at the geometric mean. Learn Motiv. 1991;22:39–58. [Google Scholar]
- Allan LG, Gerhardt K. Temporal bisection with trial referents. Percept Psychophys. 2001;63:524–40. doi: 10.3758/bf03194418. [DOI] [PubMed] [Google Scholar]
- Allen G, Muller R, Courchesne E. Cerebellar function in autism: functional magnetic resonance imaging activation during a simple motor task. Biol Psychol. 2004;56:269–78. doi: 10.1016/j.biopsych.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Allman MJ. Deficits in temporal processing associated with autistic disorder. Front Integr Neurosci. 2011;5:2. doi: 10.3389/fnint.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, Pelphrey KA, Meck WH. Developmental neuroscience of time and number: implications for autism and other neurodevelopmental diabilities. 2011b doi: 10.3389/fnint.2012.00007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, DeLeon IG. No time like the present: time perception in autism. In: Giordano AC, Lombardi VA, editors. Causes and risks for autism. New York: Nova Science Publishers; 2009. pp. 65–76. [Google Scholar]
- Allman MJ, DeLeon IG, Wearden JH. A psychophysical assessment of timing in individuals with autism. Am J Intellect Dev Disabil. 2011a;116:165–78. doi: 10.1352/1944-7558-116.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R, Ledberg A. A biologically plausible model of time-scale invariant interval timing. J Comput Neurosci. 2010;28:15–75. doi: 10.1007/s10827-009-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. A unitary model of schizophrenia. Bleuler's ‘‘fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56:781–7. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Nopoulos P, O'Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–20. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–8. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio P, Diedrichsen J, Ivry RB. Effects of focal basal ganglia lesions on timing and force control. Brain Cogn. 2005;58:62–74. doi: 10.1016/j.bandc.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Balci F, Meck WH, Moore H, Brunner D. Timing deficits in aging and neuropathology. In: Bizon JL, Wood A, editors. Animal models of human cognitive aging. Totowa, NJ: Humana Press; 2009. pp. 161–201. [Google Scholar]
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with ADHD: effects of duration, distraction and stimulant medication. J Inter Neuropsychol Soc. 1997;3:359–69. [PubMed] [Google Scholar]
- Baron-Cohen S. Theory of mind in normal development and autism. Prisme. 2001;34:174–83. [Google Scholar]
- Bauman ML, Filipek PA, Kemper TL. Early infantile autism. Inter Rev Neurobiol. 1997;41:367–86. doi: 10.1016/s0074-7742(08)60360-8. [DOI] [PubMed] [Google Scholar]
- Benke T, Delazer M, Bartha L, Auer A. Basal ganglia lesions and the theory of fronto-subcortical loops: neuropsychological findings in two patients with left caudate lesions. Neurocase. 2003;9:70–85. doi: 10.1076/neur.9.1.70.14374. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioural economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Block RA. Time and consciousness. In: Underwood G, Stevens R, editors. Aspects of consciousness, psychological issues. Vol. 1. London: Academic Press; 1979. pp. 179–217. [Google Scholar]
- Block RA. Models of psychological time. In: Block RA, editor. Hillsdale, NJ: Erlbaum; 1990. p. 1–35.
- Block RA. Psychological timing without a timer: the roles of attention and memory. In: Helfrich H, editor. Time and mind II: Information-processing perspectives. Seattle, WA: Hogrefe & Huber; 2003. pp. 41–59. [Google Scholar]
- Borst U, Cohen R. Impact of time estimation on the crossover effect in schizophrenia. Psychiatry Res. 1987;22:331–40. doi: 10.1016/0165-1781(87)90112-0. [DOI] [PubMed] [Google Scholar]
- Boucher J. ‘Lost in a sea of time’: time-parsing and autism. In: Hoerl C, McCormack T, editors. Time and memory. Oxford, UK: Oxford University Press; 2001. pp. 111–35. [Google Scholar]
- Boucher J, Pons F, Lind S, Williams D. Temporal cognition in children with autistic spectrum disorders: tests of diachronic thinking. J Aut Dev Disorder. 2007;37:1413–29. doi: 10.1007/s10803-006-0285-9. [DOI] [PubMed] [Google Scholar]
- Brannon EM, Libertus ME, Meck WH, Woldorff MG. Electrophysiological measures of time processing in infant and adult brains: Weber's law holds. J Cogni Neurosci. 2008;20:193–203. doi: 10.1162/jocn.2008.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Wolfe L, Meck WH, Woldorff M. Timing in the baby brain. Cogn Brain Res. 2004;21:227–33. doi: 10.1016/j.cogbrainres.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14:209–24. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Bschor T, Ising M, Bauer M, Lewitzka U, Skerstupeit M, Müller-Orlinghausen B, et al. Time experience and time judgment in major depression, mania and healthy subjects. A controlled study of 93 subjects. Acta Psych Scand. 2004;109:222–9. doi: 10.1046/j.0001-690x.2003.00244.x. [DOI] [PubMed] [Google Scholar]
- Bueti D, Walsh V, Frith C, Rees G. Different brain circuits underlie motor and perceptual representations of temporal intervals. J Cogn Neurosci. 2008;20:204–14. doi: 10.1162/jocn.2008.20017. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Aziz D, Winslow D, Carter RE, Swearington JE, Buhusi MC. Interval timing accuracy and scalar timing in C57BL/6 mice. Behav Neurosci. 2009;123:1102–13. doi: 10.1037/a0017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behav Neurosci. 2002;116:291–7. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–65. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Effect of clozapine on interval timing and working memory for time in the peak-interval procedure with gaps. Behav Process. 2007;74:159–67. doi: 10.1016/j.beproc.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: New findings and an extension of the resource allocation model of temporal processing. Phil Trans R Soc London B. 2009a;364:1875–85. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relativity theory and time perception: single or multiple clocks? PLoS ONE. 2009b;4:e6268. doi: 10.1371/journal.pone.0006268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonomano DV. The biology of time across different scales. Nat Chem Biol. 2007;3:594–7. doi: 10.1038/nchembio1007-594. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Maass W. State-dependent computations: Spatiotemporal processing in cortical networks. Nat Rev Neurosci. 2009;10:113–25. doi: 10.1038/nrn2558. [DOI] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, Puttfarcken PS. Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharmacology. 2005;48:72–9. doi: 10.1016/j.neuropharm.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O'Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–61. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O'Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009a;70:181–90. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Carroll CA, O'Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009b;71:345–53. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini L, Ivry RB. Effects of divided attention on temporal processing in patients with lesions of the cerebellum or frontal lobe. Neuropsychology. 1999;13:10–21. doi: 10.1037//0894-4105.13.1.10. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Ali YM, Meck WH. Ketamine “unlocks” the reduced clock-speed effect of cocaine following extended training: Evidence for dopamine-glutamate interactions in timing and time perception. Neurobiol Learn Mem. 2007a;88:149–59. doi: 10.1016/j.nlm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Dyke AG, McConnell MW, Meck WH. Categorical scaling of duration as a function of temporal context in aged rats. Brain Res. 2011;1381:175–86. doi: 10.1016/j.brainres.2011.01.044. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Etchegaray M, Meck WH. Impairments in timing, temporal memory, and reversal learning linked to neurotoxic regimens of methamphetamine intoxication. Brain Res. 2007b;1186:255–66. doi: 10.1016/j.brainres.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Hakak OL, Meck WH. Habit formation and the loss of control of an internal clock: inverse relationship between the level of baseline training and the clock-speed enhancing effects of methamphetamine. Psychopharmacology. 2007c;193:351–62. doi: 10.1007/s00213-007-0783-2. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: Implications for neurobiological models of interval timing. Pharmacol Biochem Behav. 2006;85:114–22. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Meck WH. Prenatal choline supplementation increases sensitivity to time by reducing non-scalar sources of variance in adult temporal processing. Brain Res. 2007d;1186:242–54. doi: 10.1016/j.brainres.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church RM. A concise introduction to scalar timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca-Raton, FL: CRC Press; 2003. pp. 3–22. [Google Scholar]
- Church RM, Deluty MZ. Bisection of temporal intervals. J Exp Psychol Anim Behav Process. 1977;3:216–28. doi: 10.1037//0097-7403.3.3.216. [DOI] [PubMed] [Google Scholar]
- Church RM, Meck WH, Gibbon J. Application of scalar timing theory to individual trials. J Exp Psychol Anim Behav Process. 1994;20:135–55. doi: 10.1037//0097-7403.20.2.135. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nat Rev Neurosci. 2003;4:685–91. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Wiener M, Chatterjee A. Dissociable neural systems for timing: evidence from subjects with basal ganglia lesions. PLoS ONE. 2010;5:e10324. doi: 10.1371/journal.pone.0010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Nazarian B, Vidal F. Timing, storage, and comparison of stimulus duration engage discrete anatomical components of a perceptual timing network. J Cogn Neurosci. 2008;20:2185–97. doi: 10.1162/jocn.2008.20153. [DOI] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–8. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Critchfield TS, Kollins SH. Temporal discounting: basic research and the analysis of socially important behaviour. J Appl Behav Anal. 2001;34:101–22. doi: 10.1901/jaba.2001.34-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin-Golomb A. Parkinson's disease as a disconnection syndrome. Neuropsychol Rev. 2010;20:191–208. doi: 10.1007/s11065-010-9128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD. Nonlinearities in sensitivity to time: implictions for oscillator-based representations of interval and circadian clocks. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca-Raton, FL: CRC Press; 2003. pp. 61–75. [Google Scholar]
- Danckert J, Ferber S, Pun C, Broderick C, Striemer C, Rock S, et al. Neglected time: impaired temporal perception of multisecond intervals in unilateral neglect. J Cogn Neurosci. 2007;19:1706–20. doi: 10.1162/jocn.2007.19.10.1706. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Kisley MA, Ross RG. Effects of interval duration on temporal processing in schizophrenia. Brain Cogn. 2003;52:295–301. doi: 10.1016/s0278-2626(03)00157-x. [DOI] [PubMed] [Google Scholar]
- Davalos DB, Rojas DC, Tregellas JR. Temporal processing in schizophrenia: effects of task-difficulty on behavioural discrimination and neuronal responses. Schizophrenia Res. 2011;127:123–30. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Densen ME. Time perception and schizophrenia. Percept Mot Skills. 1977;44:436–8. doi: 10.2466/pms.1977.44.2.436. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Ivry RB, Presing J. Cerebellar and basal ganglia contributions to interval timing. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca-Raton, FL: CRC Press; 2003. pp. 457–83. [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–62. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- Droit-Volet S, Meck WH. How emotions colour our perception of time. Trends Cogn Sci. 2007;11:504–13. doi: 10.1016/j.tics.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson OB, et al. Hippocampal and caudate volume reductions in antipsychotic-naïve first-episode schizophrenia. J Psychiatry Neurosci. 2010;35:95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisler H, Eisler AD, Hellström A. Psychophysical issues in the study of time perception. In: Grondin S, editor. Psychology of time. Bingley, UK: Emerald Group Publishing; 2008. pp. 75–109. [Google Scholar]
- Elsinger CL, Rao SM, Zimbelman JL, Reynolds NC, Blindauer KA, Hoffmann RG. Neural basis for impaired time reproduction in Parkinson's disease: an fMRI study. J Int Neuropsychol Soc. 2003;9:1088–98. doi: 10.1017/S1355617703970123. [DOI] [PubMed] [Google Scholar]
- Elvevag B, McCormack T, Gilbert A, Brown GDA, Weinberger DR, Goldberg TE. Duration judgments in patients with schizophrenia. Psychol Med. 2003;33:1249–61. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- Ferster CB, Skinner BF. Schedules of reinforcement. New York: Appleton-Century-Crofts; 1957. [Google Scholar]
- Fortin C, Fairhurst S, Malapani C, Morin C, Towey J, Meck WH. Expectancy in humans in multisecond peak-interval timing with gaps. Atten Percept Psychophys. 2009;71:789–802. doi: 10.3758/APP.71.4.789. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, et al. An extended multisensory temporal binding window in autism spectrum disorder. Exp Brain Res. 2010;203:381–9. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisse P. The adaptation of the child to time. In: Friedman WJ, editor. The developmental psychology of time. New York: Academic Press; 1982. pp. 113–40. [Google Scholar]
- Fraisse P. Perception and estimation of time. Ann Rev Psychol. 1984;35:1–36. doi: 10.1146/annurev.ps.35.020184.000245. [DOI] [PubMed] [Google Scholar]
- Fuchs T. The temporal structure of intentionality and its distrurbance in schizophrenia. Psychopathology. 2007;40:229–35. doi: 10.1159/000101365. [DOI] [PubMed] [Google Scholar]
- Gescheider GA. Psychophysics: the fundamentals. Mahwah, NJ: Erlbaum; 1997. [Google Scholar]
- Ghajar J, Ivry RB. The predictive brain state: timing deficiency in traumatic brain injury? Neurorehabil Neural Repair. 2008;22:217–27. doi: 10.1177/1545968308315600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber's law in animal timing. Psych Rev. 1977;84:279–325. [Google Scholar]
- Gibbon J. Origins of scalar timing. Learn Motiv. 1991;22:3–38. [Google Scholar]
- Gibbon J, Church RM. Sources of variance in an information processing theory of timing. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal cognition. Hillsdale, NJ: Erlbaum; 1984. pp. 465–88. [Google Scholar]
- Gibbon J, Church RM. Representation of time. Cognition. 1990;37:23–54. doi: 10.1016/0010-0277(90)90017-e. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Church RM. Comparison of variance and covariance patterns in parallel and serial theories of timing. J Exp Anal Behav. 1992;57:393–406. doi: 10.1901/jeab.1992.57-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Ann NY Acad Sci. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7:170–84. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Gidley Larson JC, Mostofsky SH. Motor deficits in autism. In: Tuchman R, Rapin I, editors. Autism: a neurobiological disorder of early brain development. London: Mac Keith Press; 2006. pp. 231–47. [Google Scholar]
- Gil S, Droit-Volet S. Time perception, depression and sadness. Behav Process. 2009;80:169–76. doi: 10.1016/j.beproc.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Gillig PM, Sanders RD. Psychiatry, neurology and the role of the cerebellum. Psychiatry. 2010;7:38–43. [PMC free article] [PubMed] [Google Scholar]
- Glenthoj A, Glenthoj BY, MAckeprang T, Pagsberg AK, Hemmingsen RP, Jernigan TL, et al. Basal ganglia volumes in drug-naïve first-episode schizophrenia patients before and after short-term treatment with either a typical or an atypical antipsychotic drug. Psychiatry Res Neuroimaging. 2007;154:199–208. doi: 10.1016/j.pscychresns.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Goldstone S, Lhamon WT. Studies of auditory-visual differences in human time judgment. 1. Sounds are judged longer than lights. Percept Mot Skills. 1974;39:63–82. doi: 10.2466/pms.1974.39.1.63. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizo Bull. 2008;34:944–61. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Portugal GS, Matell MS. Evidence for separate neural mechanisms for the timing of discrete and sustained responses. Brain Res. 2007;1156:139–51. doi: 10.1016/j.brainres.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Gooch CM, Wiener M, Wencil EB, Coslett HB. Interval timing disruptions in subjects with cerebellar lesions. Neuropsychologia. 2010;48:1022–31. doi: 10.1016/j.neuropsychologia.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn JA, Brett M. Impairment of beat-based rhythm discrimination in Parkinson's disease. Cortex. 2009;45:54–61. doi: 10.1016/j.cortex.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Grahn JA, McAuley JD. Neural bases of individual difference in beat perception. NeuroImage. 2009;47:1894–903. doi: 10.1016/j.neuroimage.2009.04.039. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10:R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Grondin S. Timing and time perception: a review of recent behavioural and neuroscience findings and theoretical directions. Atten Percept Psychophys. 2010;72:561–82. doi: 10.3758/APP.72.3.561. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Scerif G, Liddle PF, Batty MJ, Liddle EB, Roberts KL, et al. Effects of motivation and medication on electrophysiological markers of response inhibition in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;67:624–31. doi: 10.1016/j.biopsych.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu B-M, Cheng RK, Yin B, Meck WH. Quinpirole-induced sensitization to noisy/sparse periodic input: temporal synchronization as a component of obsessive-compulsive disorder. Neurosci. 2011a;179:143–50. doi: 10.1016/j.neuroscience.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Gu B-M, Meck WH. New persectives on Vierordt's law: memory-mixing in ordinal temporal comparison tasks. In: Vatakis A, Esposito A, Cummins F, Papadelis G, Giagkou M, editors. Time and time perception. LNAI 6789. Berlin: Springer-Verlag; 2011b. pp. 67–78. [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo SY, Jo HJ, et al. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain. 2008;131:155–64. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Galve L, Wheeler-Kingshott CAM, Altmann DR, Price G, Chu EM, Leeson VC, et al. Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biol Psychiatry. 2010;68:51–60. doi: 10.1016/j.biopsych.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé FGE. Autism: cognitive deficit or cognitive style? Trends Cogn Sci. 1999;3:216–22. doi: 10.1016/s1364-6613(99)01318-2. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Boyd LA, Mayer AR, Sheltraw DM, Lee RR, Huang M, et al. Neural representation of interval encoding and decision making. Cogn Brain Res. 2004a;21:193–205. doi: 10.1016/j.cogbrainres.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Castillo GN, Greenberg PA, Song DD, Lessig S, Lee RR, et al. Neurobehavioural mechanisms of temporal processing deficits in Parkinson's disease. PLoS ONE. 2011;6:e17461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DL, Haaland KY, Hermanowicz N. Temporal processing in the basal ganglia. Neuropsychology. 1998;12:3–12. doi: 10.1037//0894-4105.12.1.3. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Lee RR, Boyd LA, Rapcsak SZ, Knight RT. Does the representation of time depend on the cerebellum? Effect of cerebellar stroke. Brain. 2004b;127:1–14. doi: 10.1093/brain/awh065. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Zimbelman JL, Hinton SC, Rao SM. Neural modulation of temporal encoding, maintenance, and decision processes. Cerebral Cortex. 2010;20:1274–85. doi: 10.1093/cercor/bhp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Nagaoka M, Mizuno Y. Music therapy in parkinson's disease: improvement of parkinsonian gait and depression with rhythmic auditory stimulation. Parkinsonism Relat Disord. 2006;12:S76. [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psych. 2006;163:1252–63. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Höhn S, Dallérac G, Faure A, Urbach Y, Nguyen HP, Riess O, et al. Behavioral and in vivo electrophysiological evidence for presymptomatic alteration of prefronto-striatal processing in the transgenic rat model for Huntington disease. J Neurosci. 2011;31:8986–97. doi: 10.1523/JNEUROSCI.1238-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviours in autism. Biol Psychiatry. 2005;58:226–32. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Humphries MD, Lepora N, Wood R, Gumey K. Capturing dopaminergic modulation and bimodal membrane behaviour of striatal medium spiny neurons in accurate, reduced models. Front Comput Neurosci. 2009;3:26. doi: 10.3389/neuro.10.026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz M, Danckert J. Temporal discrimination of between- and within-object events in left and right space. Percept Psychophys. 2011 in press. [Google Scholar]
- Hwang S-L, Gau SS-F, Hsu W-Y, Wu Y-Y. Deficits in interval timing measured by the dual-task paradigm among children and adolescents with attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry Allied Dis. 2010;51:223–32. doi: 10.1111/j.1469-7610.2009.02163.x. [DOI] [PubMed] [Google Scholar]
- Ivry RB. The representation of temporal information in perception and motor control. Curr Opin Neurobiol. 1996;6:851–7. doi: 10.1016/s0959-4388(96)80037-7. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Schlerf JE. Dedicated and intrinsic models of time perception. Trends Cogn Sci. 2008;12:273–80. doi: 10.1016/j.tics.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Spencer RMC. The neural representation of time. Curr Opin Neurobiol. 2004;14:225–32. doi: 10.1016/j.conb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Dirnberger G, Frith CD. The substantia nigra pars compacta and temporal processing. J Neurosci. 2006;26:12266–73. doi: 10.1523/JNEUROSCI.2540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CR, Zijlmans J, Katsenschlager R, Lee L, Quinn N, et al. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson's disease. Brain. 2010a;133:727–45. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Wilkinson L, Gahir H, Dharminda A, Lagnado DA. Medication impairs probabilistic classification learning in Parkinson's disease. Neuropsychologia. 2010b;48:1096–103. doi: 10.1016/j.neuropsychologia.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Jones CR, Jahanshahi M. The substantia nigra, the basal ganglia, dopamine and temporal processing. J Neural Transm Suppl. 2009;73:161–71. doi: 10.1007/978-3-211-92660-4_13. [DOI] [PubMed] [Google Scholar]
- Jones CRG, Malone TJL, Dirnberger G, Edwards M, Jahanshahi M. Basal ganglia, dopamine and temporal processing: performance on three timing tasks on and off medication in Parkinson's disease. Brain Cogn. 2008;68:30–41. doi: 10.1016/j.bandc.2008.02.121. [DOI] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. Timing in the absence of clocks: Encoding time in neural network states. Neuron. 2007;53:427–38. doi: 10.1016/j.neuron.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Brusa L, Oliveri M, Stanzione P, Caltagirone C. Memory for time intervals is impaired in left hemi-Parkinson patients. Neuropsychologia. 2005;43:1163–7. doi: 10.1016/j.neuropsychologia.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Koch G, Costa A, Brusa L, Peppe A, Gatto I, Torriero S, et al. Impaired reproduction of second but not millesecond time intervals in Parkinson's disease. Neuropsychologia. 2008a;46:1305–13. doi: 10.1016/j.neuropsychologia.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Caltagirone C. Neural networks engaged in milliseconds and seconds time processing: evidence from transcranial magnetic stimulation and patients with cortical or subcortical dysfunction. Phil Trans R Soc B. 2009;364:1907–18. doi: 10.1098/rstb.2009.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res. 2007;179:291–9. doi: 10.1007/s00221-006-0791-1. [DOI] [PubMed] [Google Scholar]
- Koch G, Ribolsi M, Mori F, Sacchetti L, Codeca C, Rubino IA, et al. Connectivity between posterior parietal cortex and ipsilateral motor cortex is altered in schizophrenia. Biol Psychiatry. 2008b;64:815–9. doi: 10.1016/j.biopsych.2008.05.026. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–8. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Honey GD, Soni W, Bullmore ET, Williams SC, et al. Procedural learning in schizophrenia: a functional magnetic resonance imaging investigation. Schizophr Res. 2002;57:97–107. doi: 10.1016/s0920-9964(01)00270-5. [DOI] [PubMed] [Google Scholar]
- Lalli JS, Casey S, Goh H, Merlino J. Treatment of escape-maintained aberrant behaviour with escape extinction and predictable routines. J Appl Behav Anal. 1994;27:705–14. doi: 10.1901/jaba.1994.27-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Tucha O, Steup A, Gsell W, Naumann M. Subjective time estimation in Parkinson's disease. J Neural Trans. 1995;46:433–8. [PubMed] [Google Scholar]
- Lee K-H, Bhaker RS, Mysore A, Parks RW, Birkett PBL, Woodruff PWR. Time perception and its neuropsychological correlates in patients with schizophrenia and in healthy volunteers. Psychiatry Res. 2009;166:174–83. doi: 10.1016/j.psychres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Leisman G, Melillo R. Effects of motor sequence training on attentional performance in ADHD children. Int J Disabil Hum Dev. 2010 Advance Access published on August 20, 2011, doi:10.1515/ijdhd.2010.043. [Google Scholar]
- Leisman G, Melillo R, Thum S, Ransom MA, Orlando M, Tice C, et al. The effect of hemisphere specific remediation strategies on the academic performance outcome of children with ADD/ADHD. Int J Adol Med Health. 2010;22:275–83. doi: 10.1515/ijamh.2010.22.2.275. [DOI] [PubMed] [Google Scholar]
- Lejeune H, Wearden JH. Scalar properties in animal timing: conformity and violations. Quart J Exp Psychol. 2006;59:1875–908. doi: 10.1080/17470210600784649. [DOI] [PubMed] [Google Scholar]
- Lejeune H, Wearden JH. Vierordt's The Experimental Study of the Time Sense (1868) and its legacy. Eur J Cogn Psychol. 2009;21:941–60. [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–41. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, et al. Nicotine effects on adults with attention-deficit/hyperactivity disorder. Psychopharmacology. 1996;123:55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levit-Binnun N, Handzy NZ, Peled A, Modai I, Moses E. Transcranial magetic stimulation in a finger-tapping task separates motor timing mechanisms and induces frequency doubling. J Cogn Neurosci. 2007;19:721–33. doi: 10.1162/jocn.2007.19.5.721. [DOI] [PubMed] [Google Scholar]
- Lewis PA, Miall RC. The precision of temporal judgement: milliseconds, many minutes, and beyond. Phil Trans R Soc B. 2009;364:1897–905. doi: 10.1098/rstb.2009.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C, Matell MS, Meck WH. Not “just” a coincidence: frontal-striatal interactions in working memory and interval timing. Memory. 2005;13:441–8. doi: 10.1080/09658210344000404. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Paying attention to time as one gets older. Psychol Sci. 2001;12:478–84. doi: 10.1111/1467-9280.00389. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Chronic treatment with haloperidol induces working memory deficits in feedback effects of interval timing. Brain Cogn. 2005;58:9–16. doi: 10.1016/j.bandc.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Lustig C, Meck WH. Modality differences in timing and temporal memory throughout the lifespan. Brain Cogn. 2011 doi: 10.1016/j.bandc.2011.07.007. Advance Access published on August 20, 2011, doi:10.1016/j.bandc.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Macar F, Lejeune H, Bonnet M, Ferrara A, Pouthas V, Vidal F, et al. Activation of the supplementary motor area and of attentional networks during temporal processing. Exp Brain Res. 2002;142:475–85. doi: 10.1007/s00221-001-0953-0. [DOI] [PubMed] [Google Scholar]
- Macar F, Vidal F. Timing processes: an outline of behavioural and neural indicies not systematically considered in timing models. Canadian J Exp Psychol. 2009;63:227–39. doi: 10.1037/a0014457. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Systems-level integration of interval timing and reaction time. Neurosci Biobehav Rev. 2004;28:747–69. doi: 10.1016/j.neubiorev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology. 2005;182:232–44. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Interaction of raclopride and preparatory-interval effects on simple reaction-time performance. Behav Brain Res. 2006;175:62–74. doi: 10.1016/j.bbr.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Madison G. Variability in isochronous tapping: higher order dependencies as a function of intertap interval. J Exp Psychol Hum Percept Perf. 2001;27:411–22. doi: 10.1037//0096-1523.27.2.411. [DOI] [PubMed] [Google Scholar]
- Maister L, Plaisted K. Episodic autobiographical memory, time perception and self-awareness in ASD. Presented at Inter Soc Autism Res Abstr. 2009 Chicago, IL. [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–7. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson's disease. J Cogn Neurosci. 2002;14:311–22. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, et al. Coupled temporal memories in Parkinson's disease: a dopamine-related dysfunction. J Cogn Neurosci. 1998;10:316–31. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin BC. Interval timing in the dopamine-depleted basal ganglia: from empirical data to timing theory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Florida: CRC Press; 2003. pp. 485–514. [Google Scholar]
- Martin JS, Poirier M, Bowler DM. Brief report: impaired temporal reproduction performance in adults with autism spectrum disorder. J Autism Dev Disord. 2010;40:640–6. doi: 10.1007/s10803-009-0904-3. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Neuropsychological mechanisms of interval timing behaviour. Biosessays. 2000;22:94–103. doi: 10.1002/(SICI)1521-1878(200001)22:1<94::AID-BIES14>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn Brain Res. 2004;21:139–70. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MAL. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behav Neurosci. 2003;117:760–73. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- Matell MS, Shea-Brown E, Gooch C, Wilson AG, Rinzel J. A heterogeneous population code for elapsed time in rat medial agranular cortex. Behav Neurosci. 2011;125:54–73. doi: 10.1037/a0021954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Process. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Attentional bias between modalities – Effect on the internal clock, memory and decision stages used in animal time discrimination. Ann NY Acad Sci. 1984;423:528–41. doi: 10.1111/j.1749-6632.1984.tb23457.x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharm Biochem Behav. 1986;25:1185–9. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cogn Brain Res. 1996;3:227–42. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Interval timing and genomics: What makes mutant mice tick? Inter J Comp Psychol. 2001;14:211–31. [Google Scholar]
- Meck WH. Choline uptake in the frontal cortex is proportional to the absolute error of a temporal memory translation constant in mature and aged rats. Learn Motiv. 2002a;33:88–104. [Google Scholar]
- Meck WH. Distortions in the content of temporal memory: neurobiological correlates. In: Fountain SB, Bunsey MD, Danks JH, McBeath MK, editors. Animal cognition and sequential behaviour: behavioral, biological, and computational perspectives. Boston, MA: Kluwer Academic Press; 2002b. pp. 175–200. [Google Scholar]
- Meck WH. Neuropsychology of timing and time perception. Brain Cogn. 2005;58:1–8. doi: 10.1016/j.bandc.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Meck WH. Frontal cortex lesions eliminate the clock speed effect of dopaminergic drugs on interval timing. Brain Res. 2006a;1108:157–67. doi: 10.1016/j.brainres.2006.06.046. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuroanatomical localization of an internal clock: a functional link between mesolimbic, nigrostriatal, and mesocortical dopaminergic systems. Brain Res. 2006b;1109:93–107. doi: 10.1016/j.brainres.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Meck WH. Temporal memory in mature and aged rats is sensitive to choline acetyltransferase inhibition. Brain Res. 2006c;1108:168–75. doi: 10.1016/j.brainres.2006.06.047. [DOI] [PubMed] [Google Scholar]
- Meck WH, Benson AM. Dissecting the brain's internal clock: how frontal-stratial circuitry shifts time and keeps attention. Brain Cogn. 2002;48:195–211. doi: 10.1006/brcg.2001.1313. [DOI] [PubMed] [Google Scholar]
- Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Çevik MÖ. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.01.042. Advance Access published on August 20, 2011, doi:10.1016/j.neuropharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM. Abstraction of temporal attributes. J Exp Psychol Anim Behav Process. 1982;8:226–43. [Google Scholar]
- Meck WH, Church RM, Gibbon J. Temporal integration in duration and number discrimination. J Exp Psychol Anim Behav Process. 1985;11:591–7. [PubMed] [Google Scholar]
- Meck WH, MacDonald CJ. Amygdala inactivation reverses fear's ability to impair divided attention and make time still. Behav Neurosci. 2007;121:707–20. doi: 10.1037/0735-7044.121.4.707. [DOI] [PubMed] [Google Scholar]
- Meck WH, Malapani C. Neuroimaging of interval timing. Cogn Brain Res. 2004;21:133–7. doi: 10.1016/j.cogbrainres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opin Neurobiol. 2008;18:1–8. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. NeuroReport. 1997a;8:2831–5. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. NeuroReport. 1997b;8:3045–51. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. J Neuropsychiatry Clin Neurosci. 1994;6:358–70. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Melgire M, Ragot R, Samson S, Penney TB, Meck WH, Pouthas V. Auditory/visual duration bisection in patients with left or right medial-temporal lobe resection. Brain Cogn. 2005;58:119–24. doi: 10.1016/j.bandc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Melillo R, Leisman G. Autistic spectrum disorders as functional disconnection syndrome. Rev Neurosci. 2009;20:111–31. doi: 10.1515/revneuro.2009.20.2.111. [DOI] [PubMed] [Google Scholar]
- Merchant H, Luciana M, Hooper C, Majestic S, Tuite P. Interval timing and Parkinson's disease: heterogeneity in temporal performance. Exp Brain Res. 2008a;184:233–48. doi: 10.1007/s00221-007-1097-7. [DOI] [PubMed] [Google Scholar]
- Merchant H, Zarco W, Prado L. Do we have a common mechanisms for measuring time in the hundreds of millisecond range? Evidence from multiple-interval timing tasks. J Neurophysiol. 2008b;99:939–49. doi: 10.1152/jn.01225.2007. [DOI] [PubMed] [Google Scholar]
- Michon JA. The making of the present: A tutorial review. In: Requin J, editor. Attention and performance. VII. Lawrence Erlbaum, Hillsdale: NJ; 1978. pp. 89–111. [Google Scholar]
- Middleton FA, Strick PL. Cerebellar output channels. In: Schshmann JD, editor. The cerebellum and cognition. Vol. 41. San Diego, CA: Academic Press; 1997. pp. 31–60. [Google Scholar]
- Minshew NJ, Goldstein G, Siegal DJ. Neuropsychologic functioning in autism: profile of a complex information processing disorder. J Int Neuropsychol Soc. 1997;3:303–16. [PubMed] [Google Scholar]
- Montangero J, Pons F, Scheidegger P. The evolution of conceptions of biological transformations. In: Montangero J, editor. Understanding changes in time. London: Taylor & Francis; 1996. pp. 20–9. [Google Scholar]
- Mosconi MW, Kay M, D'Cruz A-M, Guter S, Kapur K, Macmillan C, et al. Neurobehavioural abnormalities in first-degree relatives of individuals with autism. Arch Gen Psychiatry. 2010;67:830–40. doi: 10.1001/archgenpsychiatry.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, et al. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Nakano T, Ota H, Kato N, Kitazawa S. Deficit in visual temporal integration in autism spectrum disorders. Proc R Soc B. 2010;277:1027–30. doi: 10.1098/rspb.2009.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. Language and the self: From the “Experiencing I” to the “Continuing Me”. In: Moore C, Lemmon K, editors. The self in time: developmental perspectives. Mahwah, NJ: Erlbaum; 2001. pp. 15–34. [Google Scholar]
- Nelson K, Skwerer DP, Goldman S, Henseler S, Presler N, Walkenfeld FF. Entering a community of minds: an experiential approach to ‘Theory of Mind’. Hum Dev. 2003;46:24–46. [Google Scholar]
- Nir I, Meir D, Zilber N, Knobler H, Hadjez J, Lerner Y. Brief report: circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J Autism Dev Disord. 1995;25:614–54. doi: 10.1007/BF02178193. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Executive function deficits in high-functioning autistic individuals: relationship to theory of mind. J Child Psychol Psych. 1991;32:1081–105. doi: 10.1111/j.1469-7610.1991.tb00351.x. [DOI] [PubMed] [Google Scholar]
- Paccehetti C, Francesca M, Aglieri R, Fundarò C, Martignoni E, Nappi G. Active music therapy in Parkinson's disease: an integrative method for motor and emotional rehabilitation. Psychosomatic Med. 2000;62:386–93. doi: 10.1097/00006842-200005000-00012. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, et al. Procedural learning in Parkinson's disease and cerebellar degeneration. Ann Neurol. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Pastor MA, Artieda J, Jahanshahi M, Obeso JA. Time estimation and reproduction is abnormal in Parkinson's disease. Brain. 1992;115:211–25. doi: 10.1093/brain/115.1.211. [DOI] [PubMed] [Google Scholar]
- Paule MG, Meck WH, McMillan DE, McClure GYH, Bateson M, Popke EJ, et al. The use of timing behaviours in animals and humans to detect drug and/or toxicant effects. Neurotox Teratol. 1999;21:491–502. doi: 10.1016/s0892-0362(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Penney TB. Modality differences in interval timing: attention, clock speed, and memory. In: Meck WH, editor. Functional and neural mechanisms of interval timing. Boca-Raton, FL: CRC Press; 2003. pp. 209–33. [Google Scholar]
- Penney TB, Allan LG, Meck WH, Gibbon J. Memory mixing in duration bisection. In: Rosenbaum DA, Collyer CE, editors. Timing of behaviour: neural, psychological and computational perspectives. Cambridge, MA: MIT Press; 1998. pp. 165–93. [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Differential effects of auditory and visual signals on clock speed and temporal memory. J Exp Psychol Hum Percept Perform. 2000;26:1770–87. doi: 10.1037//0096-1523.26.6.1770. [DOI] [PubMed] [Google Scholar]
- Penney TB, Gibbon J, Meck WH. Categorical scaling of duration bisection in pigeons (Columba livia), mice (Mus musculus), and humans (homo sapiens) Psychol Sci. 2008;19:1103–9. doi: 10.1111/j.1467-9280.2008.02210.x. [DOI] [PubMed] [Google Scholar]
- Penney TB, Holder MD, Meck WH. Clonidine-induced antagonism of norepinephrine modulates the attentional processes involved in peak-interval timing. Exp Clin Psychopharmacol. 1996;4:82–92. [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–18. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Penney TB, Tourret S. Modality effects in short interval timing. Psychologie Française. 2005;50:131–43. [Google Scholar]
- Perbal S, Deweer B, Pillon B, Vidailhet M, Dubois B, Pouthas V. Effects of internal clock and memory disorders on duration reproductions and duration productions in patients with Parkinson's disease. Brain Cogn. 2005;58:35–48. doi: 10.1016/j.bandc.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Picard H, Amado I, Mouchet-Mages S, Olie J-P, Krebs M-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull. 2008;34:155–72. doi: 10.1093/schbul/sbm049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30:8888–96. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piras F, Coull JT. Implicit perceptual timing tasks draw upon the same scalar representation of time as explicit timing tasks. PLoS ONE. 2011;6:e18203. doi: 10.1371/journal.pone.0018203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak Y, Kroyzer N, Yakir A, Friedler M. Testing possible mechanisms of deficient supra-second time estimation in adults with attention-deficit/hyperactivity disorder. Neuropsychol. 2009;23:679–86. doi: 10.1037/a0016281. [DOI] [PubMed] [Google Scholar]
- Poppel E. A hierarchical model of temporal perception. Trends Cogn Neurosci. 1997;1:56–61. doi: 10.1016/S1364-6613(97)01008-5. [DOI] [PubMed] [Google Scholar]
- Poppel E. Pre-semantically defined temporal windows for cognitive processing. Phil Trans R Soc B. 2009;364:1887–96. doi: 10.1098/rstb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouthas V, Perbal S. Time perception depends o accurate clock mechanisms as well as unimpaired attention and memory processes. Acta Neurobiologiae Experimentalis. 2004;64:367–85. doi: 10.55782/ane-2004-1520. [DOI] [PubMed] [Google Scholar]
- Rammsayer T. Neuropharmacological evidence for different timing mechanisms in humans. Q J Exp Psychol B. 1999;52:273–86. doi: 10.1080/713932708. [DOI] [PubMed] [Google Scholar]
- Rammsayer T, Classen W. Impaired temporal discrimination in Parkinson's disease: temporal processing of brief durations as and indicator of degeneration of dopaminergic neurons in the basal ganglia. Int J Neurosci. 1997;91:45–55. doi: 10.3109/00207459708986364. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Gibbon J, Penney TB, Malapani C, Hinton SC, Meck WH. Scalar expectancy theory and peak-interval timing in humans. J Exp Psychol Anim Behav Process. 1998;24:15–33. doi: 10.1037//0097-7403.24.1.15. [DOI] [PubMed] [Google Scholar]
- Rakitin BC, Scarmeas N, Li T, Malapani C, Stern Y. Single-dose levodopa administration and aging independently disrupt time production. J Cogn Neurosci. 2006;18:376–87. doi: 10.1162/089892906775990615. [DOI] [PubMed] [Google Scholar]
- Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–23. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autism: challenges for the ‘new psychophysiology’. Inter J Psychophysiol. 2006;63:164–72. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Roberts WA. Are animals stuck in time? Psychol Bull. 2002;128:473–89. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Hughes L, Ghosh BC, Eckstein D, Williams-Gray CH, Fallon S, et al. Parkinson's disease and dopaminergic therapy—differential effects on movement, reward and cognition. Brain. 2008;131:2094–105. doi: 10.1093/brain/awn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria A. Interval timing: a cerebellar model and investigation of temporal production. Unpublished Masters Thesis submitted to the Faculty of the Graduate School of the University of Colorado 2002; http://worldcat.org/oclc/52803075 (20 August 2011, date last accessed) [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–50. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Schwartze M, Keller PE, Patel AD, Katz SA. The impact of basal ganglia lesions on sensorimotor synchronization, spontaneous motor tempo, and the detection of tempo changes. Behav Brain Res. 2011;216:685–91. doi: 10.1016/j.bbr.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:613–24. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Sévigny M-C, Everett J, Grondin S. Depression, attention, and time estimation. Brain Cogn. 2003;53:351–3. doi: 10.1016/s0278-2626(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Shea-Brown E, Rinzel J, Rakitin BC, Malapani C. A firing rate model of Parkinsonian deficits in interval timing. Brain Res. 2006;1070:189–201. doi: 10.1016/j.brainres.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Smith JG, Harper DN, Gittings D, Abernethy D. The effect of Parkinson's disease on time estimation as a function of stimulus duration range and modality. Brain Cogn. 2007;64:130–43. doi: 10.1016/j.bandc.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Ivry RB. Comparison of patients with Parkinson's disease or cerebellar lesions in the production of periodic movements involving event-based or emergent timing. Brain Cogn. 2005;58:84–93. doi: 10.1016/j.bandc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Karmarkar U, Ivry RB. Evaluating dedicated and intrinsic models of temporal encoding by varying context. Phil Trans R Soc B. 2009;364:1853–63. doi: 10.1098/rstb.2009.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RM, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science. 2003;300:1437–9. doi: 10.1126/science.1083661. [DOI] [PubMed] [Google Scholar]
- Stavitsky K, McNamara P, Durso R, Harris E, Auerbach S, Cronin-Golom A. Hallucination, dreaming, and frequent dozing in Parkinson disease: impact of right-hemisphere neural networks. Cogn Behav Neurol. 2008;21:143–9. doi: 10.1097/WNN.0b013e318185e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Hum Brain Map. 2007;28:394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sysoeva OV, Tonevitsky AG, Wackermann J. Genetic determinants of time perception mediated by the serotonergic system. PLoS ONE. 2010;5:e12650. doi: 10.1371/journal.pone.0012650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelag E, Kowalska J, Galkowski T, Poppel E. Temporal processing deficits in high-functioning children with autism. Brit J Psychol. 2004;95:269–82. doi: 10.1348/0007126041528167. [DOI] [PubMed] [Google Scholar]
- Toplak ME, Dockstader C, Tannock R. Temporal information processing in ADHD: findings to-date and new methods. J Neurosci Methods. 2006;15:15–29. doi: 10.1016/j.jneumeth.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Vicario CM, Pecoraro P, Turriziani P, Koch G, Caltagirone C, Oliveri M. Relativistic compression and expansion of experiential time in the left and right space. PLoS ONE. 2008;3:e1716. doi: 10.1371/journal.pone.0001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidalaki VN, Ho M-Y, Bradshaw CM, Szabadi E. Interval timing performance in temporal lobe epilepsy: differences between patients with left and right hemisphere foci. Neuropsychologia. 1999;37:1061–70. doi: 10.1016/s0028-3932(98)00155-9. [DOI] [PubMed] [Google Scholar]
- Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. Caudate nucleus volume and cognitive performance: are they related in childhood psychopathology? Biol Psychiatry. 2006;60:942–50. doi: 10.1016/j.biopsych.2006.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Pauls D. Autism. Lancet. 2003;362:1133–41. doi: 10.1016/S0140-6736(03)14471-6. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Happé F. Time perception in autism spectrum disorders. Res Autism Spectrum Disorders. 2008;2:447–55. [Google Scholar]
- Wearden JH. Internal clocks and the representation of time. In: Hoerl C, McCormack T, editors. Time and memory: issues in philosophy and psychology. Oxford, UK: Clarendon Press; 2001. pp. 37–58. [Google Scholar]
- Wearden JH. Applying the scalar timing model to human time psychology: Progress and challenges. In: Helfrich E, editor. Time and mind II: information processing perspectives. Gottingen, Germany: Hogrefe & Huber; 2003. pp. 21–39. [Google Scholar]
- Wearden JH. Origins and development of internal clock theories of psychological time. Psychologie française. 2005;50:7–25. [Google Scholar]
- Wearden JH, Bray S. Scalar timing without reference memory? Episodic temporal generalization and bisection in humans. Quart J Exp Psychol. 2001;54B:289–309. doi: 10.1080/02724990042000173. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Lejeune H. Scalar properties in human timing: conformity and violations. Q J Exp Psychol. 2008;61:569–87. doi: 10.1080/17470210701282576. [DOI] [PubMed] [Google Scholar]
- Wiener M, Lohoff FW, Coslett HB. Double dissociation of dopamine genes and timing in humans. J Cogn Neurosci. 2011;23:2811–21. doi: 10.1162/jocn.2011.21626. [DOI] [PubMed] [Google Scholar]
- Wearden JH, Smith-Spark JH, Cousins R, Edelstyn NMJ, Cody FWJ, O'Boyle DJ. Stimulus timing by people with Parkinson's disease. Brain Cogn. 2008;67:264–79. doi: 10.1016/j.bandc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Cheng RK, Etchegaray M, Meck WH. “Speed” warps time: methamphetamine's interactive roles in drug abuse, habit formation, and the biological clocks of circadian and interval timing. Curr Drug Abuse Rev. 2008;1:203–12. doi: 10.2174/1874473710801020203. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Churan J, Paulus MP. Impaired time perception and motor timing in stimulant-dependent subjects. Drug Alcohol Depend. 2007a;90:183–92. doi: 10.1016/j.drugalcdep.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007b;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trend Cogn Sci. 2008;12:7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillacourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. NeuroImage. 2007;35:222–33. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarco W, Merchant H, Prado L, Mendez JC. Subsecond timing in primates: comparison of interval production between human subjects and rhesus monkeys. J Neurophysiol. 2009;102:3191–202. doi: 10.1152/jn.00066.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]