Figure 2.
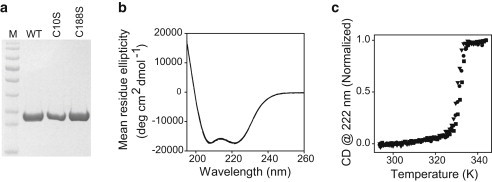
(a) SDS-PAGE of the purified WT dystrophin N-ABD and its two single-cysteine mutants C10S and C188S. Lane M represents the molecular mass markers (bottom to top: 17, 26, 34, 43, 56, 72, 95, 130, and 170 kDa, respectively). (b) CD spectrum of the WT and two mutants (5 μM). Note that all three spectra essentially overlap. (c) Thermal melts of the WT and two mutants (5 μM). As shown, cysteine mutations did not affect the protein structure (panel b) or stability (panel c).
