Figure 4.
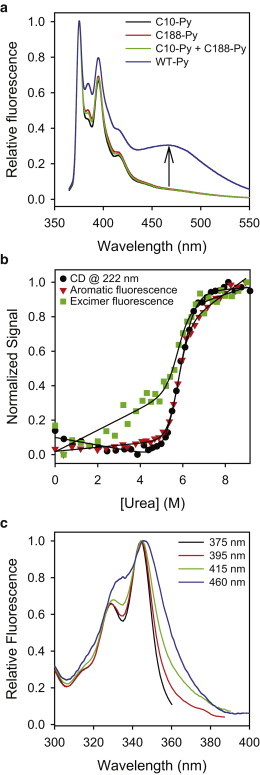
Dystrophin N-ABD is in a closed conformation in solution. (a) Pyrene fluorescence of the WT protein (WT-Py; 10 μM) in comparison with that of the single-cysteine mutants, C188S (C10-Py; 10 μM), C10S (C188-Py; 10 μM), and 1:1 C188S + C10S (C10-Py + C188-Py; 10 μM total protein concentration). The arrow denotes the excimer fluorescence of the WT protein, indicating that the protein is in a closed conformation with two cysteines close in space. (b) Urea melting of pyrene excimer fluorescence, in comparison with that measured by CD at 222 nm and aromatic fluorescence (λex = 280 nm) of the unlabeled protein. The protein concentration used in these experiments was 5 μM. All three signals measured the same protein stability (ΔG = 10.8 ± 0.8 kcal/mol, m = −1.9 ± 0.1 kcal/mol/M [urea]) when fit to a 2-state Santoro-Bolen equation (48,49), indicating that the pyrene labeling did not affect the protein structure. (c) Excitation spectra at different emission wavelengths. The spectrum corresponding to the excimer fluorescence (λem = 460 nm) is much broader than that of the monomer fluorescence (λem = 375 nm), indicating that the excimer fluorescence originates from both ground-state pyrene monomers as well as dimers.
