Figure 5.
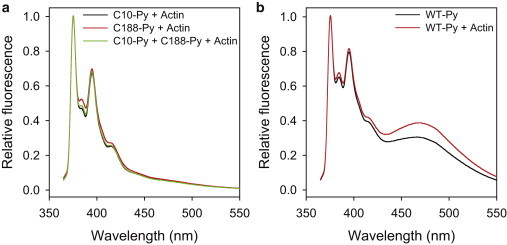
Dystrophin N-ABD is in a closed conformation when bound to F-actin. Panel (a) shows the pyrene fluorescence of the two single-cysteine mutants, C188S (C10-Py; 10 μM), C10S (C188-Py; 10 μM), and 1:1 C10-Py + C188-Py (10 μM protein concentration), whereas panel (b) shows the pyrene fluorescence of the WT N-ABD (10 μM), respectively, upon binding to F-actin (10 μM). WT protein shows excimer fluorescence, indicating that it is in a closed conformation when bound to F-actin. For these experiments, the single-cysteine mutants were labeled by 30%, whereas the WT protein was labeled such that the fraction of the doubly labeled protein was 10%.
