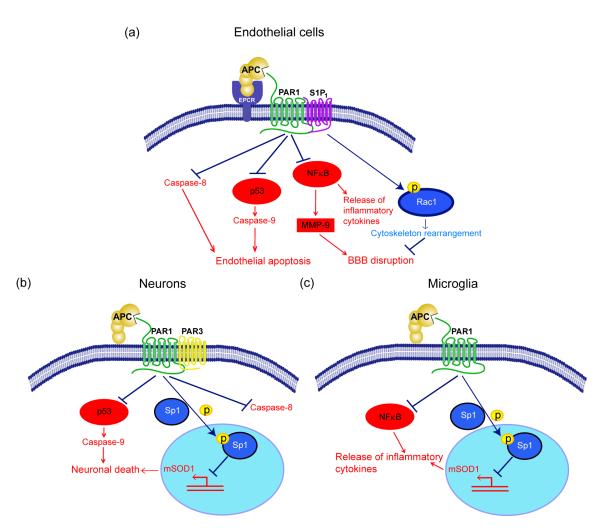
Figure 3. APC protective signaling activities in different cell types within the neurovascular unit.
a. BBB ‘sealing’ effect and direct vasculoprotection: Beneficial cytoprotective activities of APC and its cytoprotective-selective variants involve direct effects on endothelial cells that require the cellular receptors EPCR and PAR-1 and EPCR-dependent PAR1-mediated cross-activation of sphingosine 1-phosphate receptor 1 (S1P1). Cross-activation of S1P1 activates Rac1 leading to Rac1-dependent stabilization of the cytoskeleton, which results in enhanced integrity of the endothelial membranes (right). APC also suppresses NF-kB-dependent transcriptional activation of MMP-9 which in turn blocks degradation of the BBB basement membrane, thereby preventing intracerebral bleeding (middle right). By controlling Nf-κB nuclear translocation, APC blocks expression of pro-inflammatory cytokines, thereby limiting neuroinflammation (middle left). By activating PAR-1 in a EPCR-dependent manner, APC suppresses the pro-apoptotic p53 transcription factor and p53-dependent transcription of Bax (not shown) and directly upregulates anti-apoptotic Bcl-2 (not shown), which results in blockade of caspase-9 activation and controls the intrinsic apoptotic pathway (middle left). APC can also block the extrinsic apoptotic pathway and caspase-8 activation (left). b. Direct neuronal protection: APC and its cytoprotective cell signaling variants act through PAR-1 and PAR-3 to inhibit p53 activation in injured neurons resulting in blockade of caspase-9-dependent intrinsic apoptotic pathway (left) and caspase-8-dependent extrinsic apoptotic pathway (right). APC also blocks nuclear transport of the transcription factor, Sp1, through phosphorylation of cytoplasmic Sp1 resulting in transcriptional suppression of mutant SOD1 (mSOD1) expression in motor neurons in a mouse ALS model (middle). c. Anti-inflammatory activity: In SOD1 mutant mice, APC and its cell signaling variants downregulate mSOD1 expression though PAR-1 and the downstream mechanisms as noted in panel b. This suppresses microglia activation resulting in reduced number of activated microglia as well as blockade of inflammatory cytokine production from microglia (right). On the left is shown that APC blocks NF-κB activation in microglia providing another anti-inflammatory pathway mediated by a blockade of NF-kB-dependent transcriptional expression of different pro-inflammatory cytokines as noted above in a. for endothelial cells.