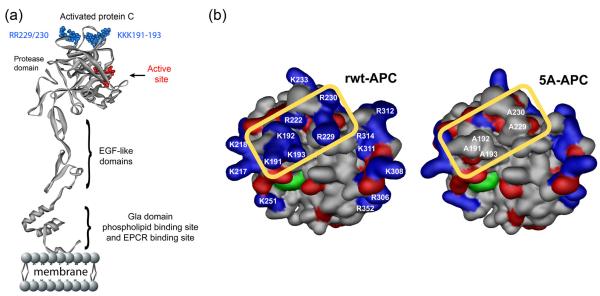
Figure I. APC exosite structures responsible for APC activities.
a. The model of full-length APC is based on the serine protease domain crystal structure of APC (105,106; Protein Data Bank entry 1AUT; http://www.rcsb.org/pdb/explore/explore.do?structureId=1AUT). In the ribbon diagram of the polypeptide structure of APC, the folded serine protease domain at the top of panel, the two EGF-like domains and the N-terminal Gla-domain (which mediates binding to phospholipid membranes and EPCR) are shown in gray. The active serine protease site is in red. The positively charged lysine residues within the so-called 37 loop (KKK191-193) and the arginine residues in the calcium binding-binding loop (RR229/230) determine APC specificity for factors Va and VIIIa.
b. Amino acid residues that determine exosite specificity of the recombinant wild-type APC (wt-APC) protease domain are schematically identified in the space filling model where the close apposition of the 37-loop and the calcium-binding loop positively charged residues (blue) comprising lysine residues K191-193, and arginine residues R229 and R230 are seen inside the yellow rectangle. The active site triad of serine, histidine and aspartic acid residues characteristic of serine proteases is shown in green while red indicates negative side chains. These five basic residues are critical for recognition of the coagulation factor Va and thus for anticoagulant activity; however, they are not required for normal anti-apoptotic activity. The 5A-APC mutant (containing 5 Ala substitutions at residues 191-193 and 229-230) is depicted on the right side inside the yellow rectangle showing a remarkable loss of the positively charged amino acid side chain cluster. The model of wt-APC is based on the serine protease domain structure of APC 1AUT (107) and was generated using Modeller (107).