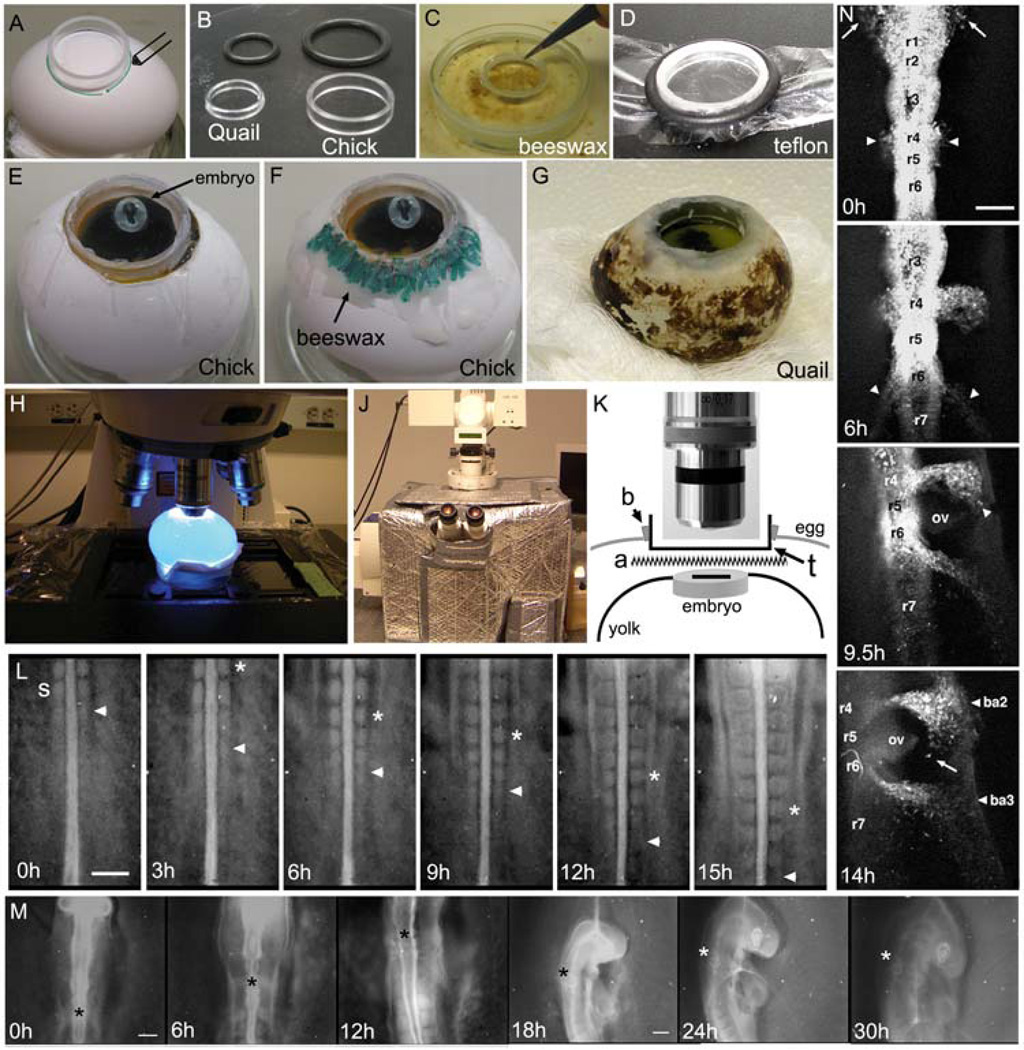
Fig. 1. In ovo culture and imaging of chick embryogenesis.
(A–G) The initial steps of in ovo imaging begin with marking the position of the presumptive window in the eggshell and creation of the teflon membrane that will fit into the window to provide an optical pathway to the embryo. (A) An acrylic ring is placed and marked over the position of the embryo (determined by candling) to create a window into the eggshell. (B) The ring diameter can vary depending on the size of the egg or embryo. We show the quail and chick appropriate rings and rubber o-rings used to stretch the teflon membrane over the ring. (C) The ring is first dipped into melted beeswax before (D) placing the ring onto the Teflon membrane and securing in tightly with the rubber o-ring, around its circumference. (E) After stretching and cutting the teflon to fit tightly over the ring, the ring is placed into the hole in the eggshell and laid over the embryo (the embryo contrast has been adjusted to better visualize its position). (F) Warmed beeswax (highlighted in green) is spread around the ring to seal it into the eggshell. (G) The same method can be applied to eggs of various sizes, including the smaller quail egg. (H) The egg is placed on the microscope stage under the objective (highlighted with blue excitation light), which (J) is surrounded by a heated chamber. (K) The optical pathway through the teflon window includes the teflon membrane (t), laid above the albumen and Ringer’s solution (a) that sits over the embryo on top of the yolk surface. (L) A typical in ovo time-lapse imaging session of somitogenesis shows the consistent addition of somites (2somites/3hrs(h)). The last forming somite is marked by the arrowhead and the 2 somites formed in between each frame is marked by the asterisk. The time-lapse shows individual frames up to 15hrs of a 24hr long session. The embryo was unlabeled and the image was created by opening the shutter of a widefield microscope at 3min intervals. The reflected light image was enhanced by a low light level light source shined onto the teflon surface. (M) A typical in ovo time-lapse of head morphogenesis reveals the tremendous amount of growth and movement (rotation) of the early chick embryo (compare the size of the head at 0h and 30h). The image sequence is shown over 30hrs of a 36hr time-lapse session and the asterisk marks the axial level of rhombomere 4. After 12hrs, the growth of the embryo required the change to a lower magnification objective (5X to 2.5X). The embryo was not labeled and images were collected in the same manner as in (L). (N) A fluorescently labeled (DiI) embryo is followed using in ovo time-lapse imaging and reveals the migration of cranial and post-otic neural crest cells emerging from both sides of rhombomere 1(r1, arrows) and r4 (arrowheads) at 0h. By 6h, neural crest cells emerge from r6 (arrowheads) and (9.5h) as the embryo rotates, neural crest cells begin to reach the peripheral branchial arches near the front of the migratory stream (arrowhead). At 14hrs, the embryo has rotated to reveal the position of the branchial arches (ba2 and ba3) and otic vesicle (ov). Individual migratory neural crest cells are seen moving at the migratory front (arrow). The scalebars are 200um (L,M,and N).