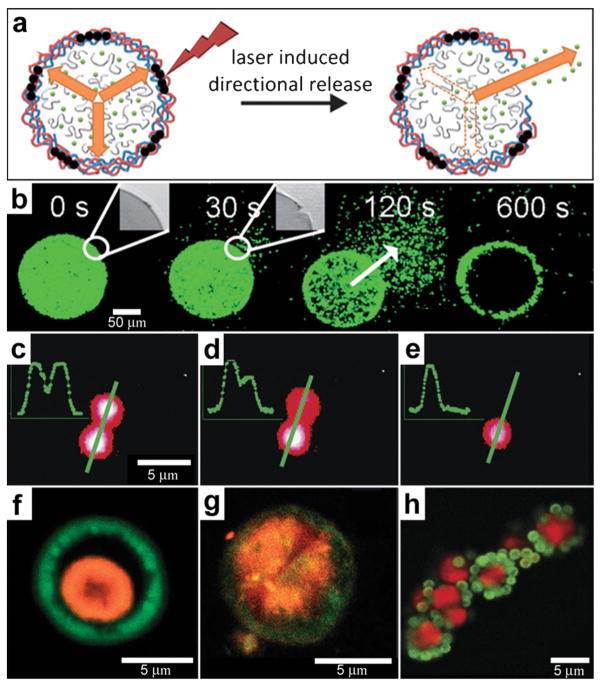
Fig. 12.
Chemical patterning with optically responsive particles. (a) Schematic representation of the laser-induced opening of a capsule at a desired area and the release of encapsulated material in a pre-selected direction. The degradation products of the dex-HEMA/DMAEMA hydrogel onto the polyelectrolyte membrane exert an osmotic pressure against the capsule wall (orange arrows). The large size of the capsules and the presence of IR-sensitive gold nanoparticles (black dots) make it easy to open the shells at a desired site using an IR laser. Once an incision is made, the content of the capsules is released. (Reprinted from ref. 130 with permission from The Royal Society of Chemistry). (b) Fluorescence microscopy snapshots of the site-specific opening of a giant polyelectrolyte capsule by IR laser activation. The inset shows the pore in the polyelectrolyte shell. The arrow indicates the direction of release as osmotic pressure drives encapsulated material out of the capsule. (Reprinted from ref. 130 with permission from The Royal Society of Chemistry). (c–e) A polymeric microcapsule shell acts as a reversible nanomembrane. Upon laser light illumination one of the microcapsules (top) partially releases encapsulated polymers (c) and reseals (d). After the second illumination the microcapsule completely releases its content (e). Profiles in the left upper corner are drawn along the green line. (Reprinted with permission from ref. 132. Copyright (2008) by The American Chemical Society). (f, g) CLSM images taken before (f) and after (g) laser-illumination of the inner shell (orange) doped with gold particles in the course of laser stimulated mixing of both compartments (outer compartment shown in green). (Reprinted with permission from ref. 134. Copyright (2007) by John Wiley and Sons). (h) Fluorescence CLSM images of pericentric multicompartment structures based on the CaCO3 inner core (red) and PS nanoparticles in the outer (green). (Reprinted with permission from ref. 133. Copyright (2010) by John Wiley and Sons).