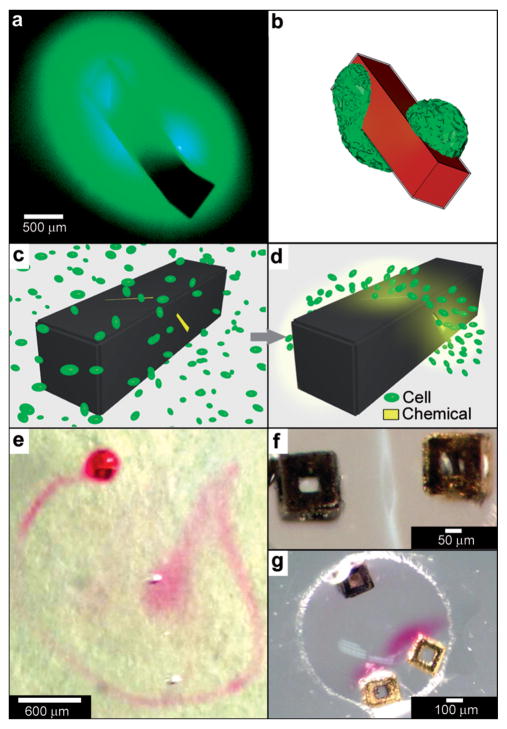
Fig. 9.
Curved, anisotropic and dynamic chemical patterns created by self-assembled microcontainers. (a, b) Generation of 3D spatial patterns by varying pore placement on self-assembled microcontainers. Experimental optical image (a) and numerical simulation (b) of the helical spatial pattern of fluorescein emerging from the container. (Reprinted with permission from ref. 113. Copyright (2011) by John Wiley and Sons). (c, d) Conceptual representation of chemotactic self-organization of motile cells in the shape of the underlying chemical pattern. At the start of the experiment, the chemoattractant is confined to the container, and the cells (represented by green ellipsoids) are distributed uniformly throughout the medium (c). The cells then self-organize in a helical pattern based on the chemical pattern once the chemoattractant (yellow) is allowed to diffuse out of the container (d). (Reprinted with permission from ref. 113. Copyright (2011) by John Wiley and Sons). (e) Optical images showing remotely guided spatially controlled chemical pattern. In this case the container was remotely guided using magnetic fields. The letter G was formed by the direct writing of phenolphthalein in an alkaline water-glycerol medium. (Reprinted with permission from ref. 114. Copyright (2006) by The American Chemical Society). (f) Spatially controlled chemical reactions between multiple containers: reaction of copper sulfate and potassium hydroxide in an aqueous medium resulting in the formation of copper hydroxide along the central line between the containers. (Reprinted with permission from ref. 114. Copyright (2006) by The American Chemical Society). (g) Reaction of phenolphthalein (diffusing out of the two bottom containers) and potassium hydroxide (diffusing out of the top container) in an aqueous medium. (Reprinted with permission from ref. 114. Copyright (2006) by The American Chemical Society).