Abstract
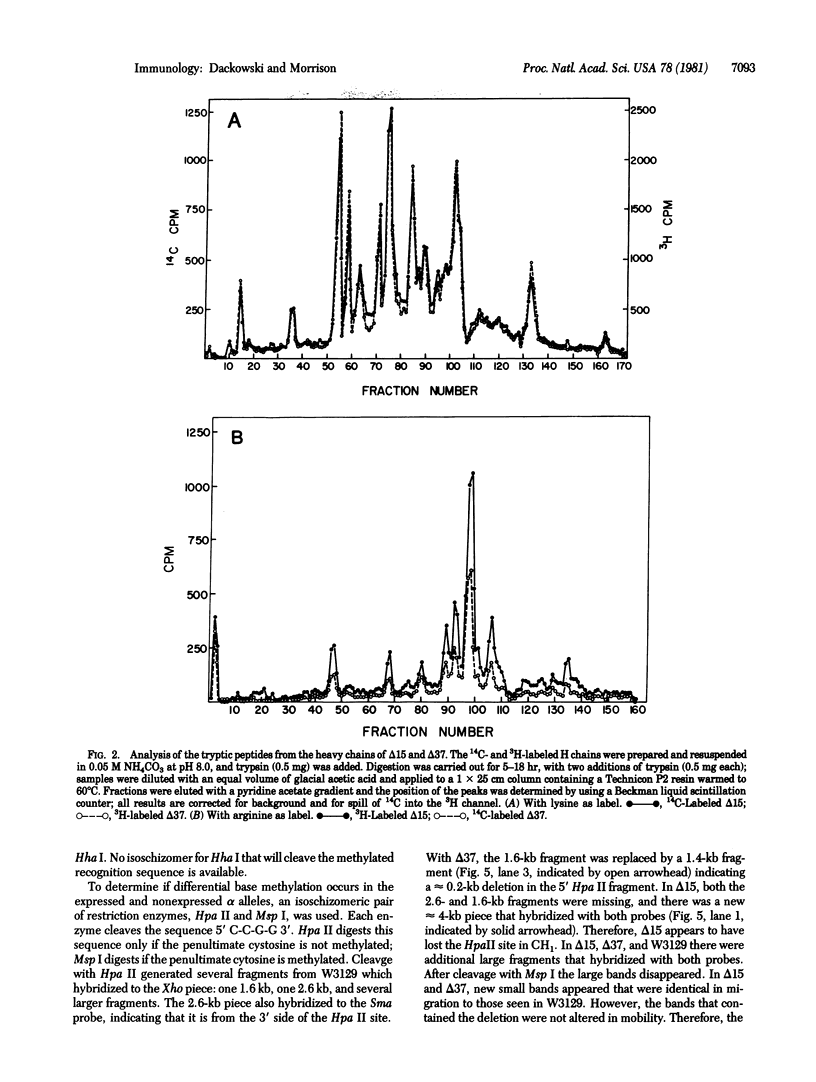
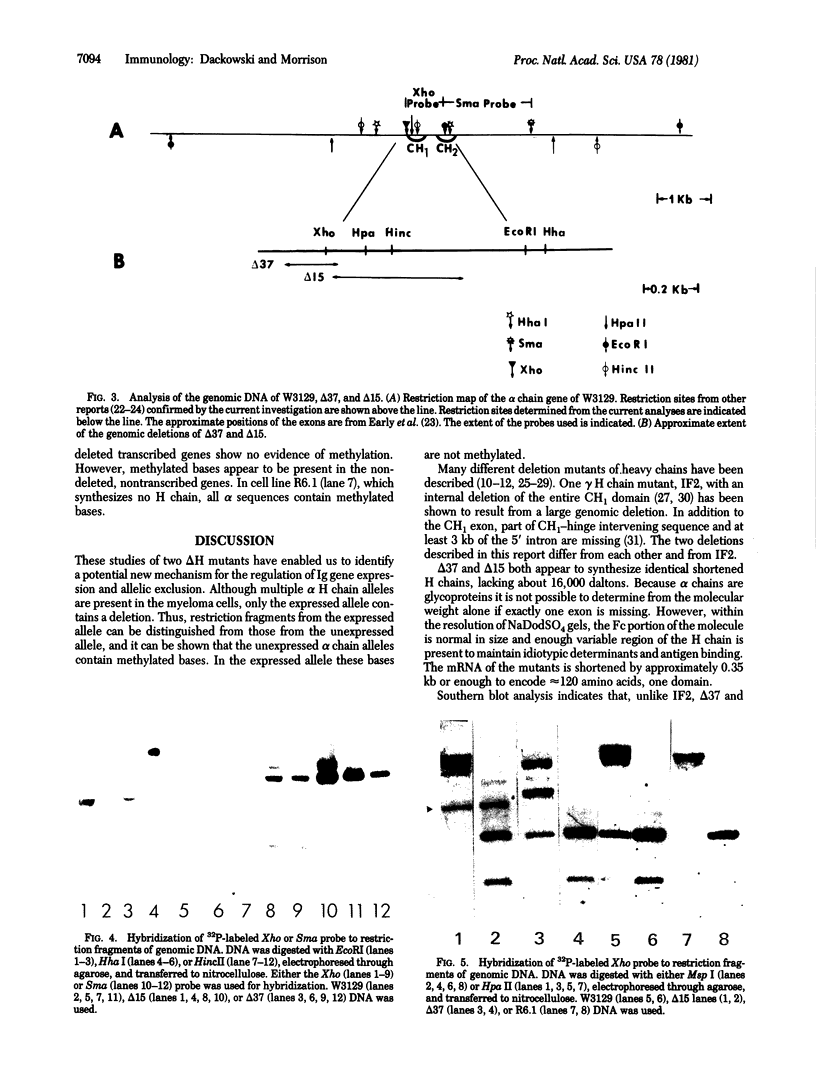
Two independently arising alpha heavy chain mutants have been found to synthesize heavy chains with CH1 deletions of approximately equal extent. Both were isolated from heavy chain-producing variants of the mouse myeloma W3129 and demonstrate that it is possible to arrive at the heavy chain disease phenotype by the pathway H + L leads to H leads to delta H. Analysis of genomic DNA by digestion with restriction endonucleases followed by molecular hybridization showed that one mutant (delta 37) had a deletion of approximately 0.2 kilobase and the second mutant (delta 15) had a deletion of approximately 0.5 kilobase. Mouse myeloma cells contain several alpha chain alleles but only one is expressed; the presence of the deletion in delta 37 and delta 15 made it possible to identify the restriction fragments from the expressed allele. Analysis of the fragments produced after cleavage with an isoschizomeric pair of restriction enzymes, Msp I and Hpa II, indicated that, in the W3129 cell line and its variants, the unexpressed alpha alleles contain methylated bases. The influence of methylation on gene expression remains to be elucidated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adetugbo K., Milstein C., Secher D. S. Molecular analysis of spontaneous somatic mutants. Nature. 1977 Jan 27;265(5592):299–304. doi: 10.1038/265299a0. [DOI] [PubMed] [Google Scholar]
- Adetugbo K., Milstein C. Spontaneous somatic frameshift mutation in a mouse myeloma. J Mol Biol. 1978 May 15;121(2):239–254. doi: 10.1016/s0022-2836(78)80007-2. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Enea V., Bothwell A. L., Baltimore D. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980 Aug;21(1):1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Choi E., Kuehl M., Wall R. RNA splicing generates a variant light chain from an aberrantly rearranged kappa gene. Nature. 1980 Aug 21;286(5775):776–779. doi: 10.1038/286776a0. [DOI] [PubMed] [Google Scholar]
- Coffino P., Baumal R., Laskov R., Scharff M. D. Cloning of mouse myeloma cells and detection of rare variants. J Cell Physiol. 1972 Jun;79(3):429–440. doi: 10.1002/jcp.1040790313. [DOI] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Dunnick W., Rabbitts T. H., Milstein C. An immunoglobulin deletion mutant with implications for the heavy-chain switch and RNA splicing. Nature. 1980 Aug 14;286(5774):669–675. doi: 10.1038/286669a0. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Franklin E. C., Frangione B. Structural variants of human and murine immunoglobulins. Contemp Top Mol Immunol. 1975;4:89–126. doi: 10.1007/978-1-4615-8930-3_4. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Morrison S. L. Murine heavy chain disease. Eur J Immunol. 1978 Mar;8(3):194–199. doi: 10.1002/eji.1830080311. [DOI] [PubMed] [Google Scholar]
- Mushinski J. F. Gamma-A half molecules: defective heavy chain mutants in mouse myeloma proteins. J Immunol. 1971 Jan;106(1):41–50. [PubMed] [Google Scholar]
- Nakhasi H. L., Lynch K. R., Dolan K. P., Unterman R. D., Feigelson P. Covalent modification and repressed transcription of a gene in hepatoma cells. Proc Natl Acad Sci U S A. 1981 Feb;78(2):834–837. doi: 10.1073/pnas.78.2.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottenburg C., Weissman I. L. Cmu gene rearrangement of mouse immunoglobulin genes in normal B cells occurs on both the expressed and nonexpressed chromosomes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):484–488. doi: 10.1073/pnas.78.1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Kearney J. F. Organization and expression of immunoglobulin genes in fetal liver hybridomas. Proc Natl Acad Sci U S A. 1981 Jan;78(1):247–251. doi: 10.1073/pnas.78.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., Coleclough C., Seidman J. G., Leder P., Tonegawa S., Matthyssens G., Weigert M. Transcription of mouse kappa chain genes: implications for allelic exclusion. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1937–1941. doi: 10.1073/pnas.77.4.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson E. A., Appella E. Amino acid sequence of a mouse myeloma immunoglobin heavy chain (MOPC 47 A) with a 100-residue deletion. J Biol Chem. 1979 Nov 25;254(22):11418–11430. [PubMed] [Google Scholar]
- Robinson E. A., Ferrini U., Seidman J. G., Appella E. Messenger RNA coding for the deleted heavy chain of mouse myeloma MOPC 47A immunoglobulin. J Biol Chem. 1980 Jun 10;255(11):4988–4991. [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Rogers J. H., Hüppi K., Brack C., Traunecker A., Maki R., Wall R., Tonegawa S. Domains and the hinge region of an immunoglobulin heavy chain are encoded in separate DNA segments. Nature. 1979 Feb 22;277(5698):627–633. doi: 10.1038/277627a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980 Aug 21;286(5775):779–783. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- Seligmann M., Mihaesco E., Preud'homme J. L., Danon F., Brouet J. C. Heavy chain diseases: current findings and concepts. Immunol Rev. 1979;48:145–167. doi: 10.1111/j.1600-065x.1979.tb00302.x. [DOI] [PubMed] [Google Scholar]
- Sutter D., Doerfler W. Methylation of integrated adenovirus type 12 DNA sequences in transformed cells is inversely correlated with viral gene expression. Proc Natl Acad Sci U S A. 1980 Jan;77(1):253–256. doi: 10.1073/pnas.77.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantravahi U., Guntaka R. V., Erlanger B. F., Miller O. J. Amplified ribosomal RNA genes in a rat hepatoma cell line are enriched in 5-methylcytosine. Proc Natl Acad Sci U S A. 1981 Jan;78(1):489–493. doi: 10.1073/pnas.78.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]