Abstract
Background
The aim of the present study was to appraise the antimicrobial activity of Ricinus communis L. essential oil against different pathogenic microorganisms and the cytotoxic activity against HeLa cell lines.
Methods
The agar disk diffusion method was used to study the antibacterial activity of Ricinus communis L. essential oil against 12 bacterial and 4 fungi strains. The disc diameters of zone of inhibition (DD), the minimum inhibitory concentrations (MIC) and the concentration inhibiting 50% (IC50) were investigated to characterize the antimicrobial activities of this essential oil. The in vitro cytotoxicity of Ricinus communis L. essential oil was examined using a modified MTT assay; the viability and the IC50 were used to evaluate this test.
Results
The essential oil from the leaves of Ricinus communis L. was analyzed by GC–MS and bioassays were carried out. Five constituents of the oil were identified by GC–MS. The antimicrobial activity of the oil was investigated in order to evaluate its efficacy against twelve bacteria and four fungi species, using disc diffusion and minimum inhibitory concentration methods. The essential oil showed strong antimicrobial activity against all microorganisms tested with higher sensitivity for Bacillus subtilis, Staphylococcus aureus and Enterobacter cloacae. The cytotoxic and apoptotic effects of the essential oil on HeLa cell lines were examined by MTT assay. The cytotoxicity of the oil was quite strong with IC50 values less than 2.63 mg/ml for both cell lines.
Conclusion
The present study showed the potential antimicrobial and anticarcinogenic properties of the essential oil of Ricinus communis L., indicating the possibilities of its potential use in the formula of natural remedies for the topical treatment of infections.
Keywords: R. communis, Essential oil, Antimicrobial activity, Cytotoxicity
Background
Essential oils obtained from aromatic plants have recently gained popularity and scientific interest. Many plants are used for different industrial purposes such as food, drugs, and perfumery manufacturing [1-3]. These compounds possess a wide spectrum of pharmacological activities [4]. They also do not enhance the “antibiotic resistance”, a phenomenon caused by long-term use of synthetic antibiotics. However, due to an increasing use of herbal products, a special care should be given to their safety, effectiveness, and drug interactions.
The dramatic increase of infectious diseases, especially those caused by microbial contamination of foods, has become, particularly in underdeveloped countries, an urgent priority [5-9]. Accordingly, there is a need to develop alternative antimicrobial drugs for their treatment. The use of local medicinal plants for possible antimicrobial and antifungal applications represents a serious promise to satisfy this need. Recently, there has been an increasing interest in essential oils as potential source of natural and safe antioxidants for food industry [5,6,9-11].
Ricinus communis (Euphorbiaceae family) is a soft wooden small tree developed throughout tropics and warm temperature regions. Having an antimicrobial activity, this plant was used to cure different ailments. It also inhibits the mitochondrial respiratory chain reactions [12]. Its leaf, root, and seed oil represent a therapeutic potential including inflammation treatment, liver disorders, hypoglycemic, and laxative [13-15]. In Tunisia, Ricinus communis is used as a contraceptive herbal drug. In addition, it is a traditional folk medicine used in the treatment of warts, cold tumors, and indurations of mammary glands, corns, and moles [16-18]. Previous studies e.g., [19] proved the anti-inflammatory and the free radical scavenging activity. It was also reported that the essential oil of this plant possesses a moderate antioxidant activity [20]. However, the antimicrobial and cytotoxic properties of R. communis essential oil have not yet been explicitly discussed.
In the present study, we investigated the antibacterial and the antifungal activities of R. communis essential oil against several pathogenic microorganisms. We also studied its antiproliferative properties against HeLa cell lines.
Methods
Chemicals, reagents and plant material
Chemicals and reagents were supported by Prolabo (Paris, France) and Pharmacia (Uppsala, Swedeen). The aerial part (leaves) of R. communis was collected during the beginning flowering stage in April 2009 from the region of Guergour, Sfax (south of Tunisia) (Figure 1). The plant materials were confirmed by A. Bekir. Voucher specimens were deposited at ISET, Sfax (Département de Génie des procédés) as Bekir 231.
Figure 1.
Geographical localization of the site of Tunisian R. communis population (from the region of Guergour, Sfax, south of Tunisia).
Distillation of essential oil and GC/MS analysis conditions
The fresh aerial parts of R. communis (300 g) were hydrodistilled using a Clevenger-type apparatus to recover the essential oils for 4 h. The distilled essential oils were dried over anhydrous sodium sulfate, filtered, and stored at +4°C.
The R. communis essential oil was analyzed using an Agilent-Technologies 6890N Network GC system equipped with a flame ionization detector and HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm; Agilent-Technologies, Little Falls, CA, USA). The injector and detector temperatures were set at 250 and 280°C, respectively. The column temperature was programmed from 35 to 250°C at a rate of 5°C/min, with the lower and upper temperatures being held for 3 and 10 min, respectively. The flow rate of the carrier gas (helium) was 1.0 ml/min. A sample of 1.0 μl was injected, using split mode (split ratio, 1:100). All quantifications were carried out using a built-in data-handling program provided by the manufacturer of the gas chromatograph. The composition was reported as a relative percentage of the total peak area. The identification of the essential oil constituents was based on a comparison of their retention times to n-alkanes, compared to published data and spectra of authentic compounds. Compounds were further identified and authenticated using their mass spectra compared to the Wiley version 7.0 library.
Antimicrobial activity assay
Microbial strain
The antimicrobial activity of the R. communis essential oil was investigated against sixteen of pathogenic microbial strains. The test microorganisms used for antimicrobial sensitivity testing including twelve species of bacteria (Staphylococcus aureus 1327, Staphylococcus epidermidis, Micrococcus luteus, Enterococcus faecalis, Enterobacter cloacae, Staphylococcus aureus 25923, Bacillus subtilis, Bacillus cereus, Pseudomonas aeruginosa 27853, Klebsiella pneumoniae WHO24, Escherchia coli 25922) and four species of fungi (Botrytis cinerea, Fusarium solani, Penicillium digitatum and Aspergillus niger) were used in this study.
Agar diffusion method
The agar diffusion method was used for the determination of antibacterial activities of R. communis essential oil according to the method described by Berghe and Vlietinck (1991) [21]. Prior to analysis, the essential oil was dissolved in absolute ethanol to create final concentration of 0.1 mg/ml and sterilized by filtration trough 0.22 μm Nylon membrane filter. Different concentrations of the R. communis essential oil were used to set a correlation between oil activity and its dose. The bacterial strains were cultured in a nutriment broth for 24 hours. Then, 200 μl of each suspension bacteria (106CFU estimated by absorbance at 600 nm) was spread on Luria Broth agar. Wells were made by using a sterile borer and were loaded with 10 μl of each sample extract. Ampicillin (10 μg/well) was used as positive control. All plates were incubated at 37°C for 24 hours. Antibacterial activity was evaluated by measuring the zone of inhibition in millimetres. All experiments were carried out in triplicates.
Determination of the minimal inhibitory concentration (MIC)
The Minimal Inhibitory Concentration (MIC) was obtained by a broth microdilution method [22] testing, which was based on reference method M38-P recommended by the NCCLS [23]. The inoculum of each bacterium was prepared and the suspensions were adjusted to 106CFU/ml. Essential oil was dissolved in absolute ethanol. Then, diluted series were prepared in a 96-well plate. Each well of the microplate included 40 μl of the growth medium, 10 μl of inoculums and 50 μl of the diluted sample of oil. The ampicillin and ethanol were used as positive and negative controls, respectively. Plates were then covered with the sterile plate and incubated at 37°C for 24 h. Subsequently, 40 μl of 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) at a final concentration 0.5 mg/ml freshly prepared in water was added to each well and incubated for 30 min. The change to red colour indicated that the bacteria are biologically active. The MIC was taken to the well, where no change of colour of MTT was observed. The experiments values were carried out in triplicate.
Antibacterial assay disc-diffusion method
All tests were performed in MHB supplemented with ethanol 5% [24,25]. Bacterial strains were cultured overnight in MHB at 37°C. Tubes of MHB containing various concentrations of essential oil were inoculated with 10 μl bacterial inoculums adjusted to 106CFU/ml. They were incubated under shaking conditions (100-120 rpm) at 37°C for 24 h [26,27]. Control tubes without tested samples were simultaneously assayed. The assays were performed in triplicate.
Antifungal assay disc-diffusion method
The biological activity against fungi was determined by employing disc agar diffusion method using Sabouraud Dextrose agar [28]. The R. communis essential oil was deposited on sterile paper discs (6 mm diameter) which were subsequently placed in the centre of the inoculated Petri dishes. After an incubation period of the 24 h at 30°C, the inhibitory activity was compared to that of commercial cycloheximide at a concentration of 1 mg/ml.
Cell lines and culture condition
HeLa cells (cervical cancer line, adherent) were used to investigate the cytotoxicity effect of R. communis essential oil. This cell line were grown in RPMI 1640 medium (Gibco) supplemented with 10% (v/v) foetal calf serum (FCS) and 2 mM L-glutamin in tissue culture flasks (Nunc). They were passed twice a week and kept at 37°C in a humidified atmosphere of 95% air and 5% CO2.
MTT test
The proliferation rates of HeLa cells after treatment with R. communis essential oil were determined by the colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The yellow compound MTT is reduced by mitochondrial dehydrogenases to the water-insoluble blue compound formazan, depending on the viability of cells.
HeLa cells (4 × 104 in each well) were incubated in 96-well plates for 24 hours in the presence or absence of essential oil. Twenty microlitres MTT solution (Sigma) (5 mg mL-1 in PBS) were added to each well. The plate was incubated for 4 h at 37°C in a CO2-incubator. One hundred and eighty microlitres of medium was removed from every well without disturbing the cell clusters. A 180 μl methanol/DMSO solution (50:50) was added to each well, and the preparations were thoroughly mixed on a plate shaker with the cell containing formazan crystals. After the dissolution of all crystals, the A570 values were determined [29] with a microplate reader (ELx 800).
Results and discussion
Antimicrobial assays
The used bacteria and fungi were selected because they are implicated with skin, oral and intestinal tract of man. The in-vitro antibacterial activity of R. communis essential oil was evaluated by a paper disc diffusion method against sixteen microorganisms. Their potency was assessed quantitatively by Disc Diameters (DD) of inhibition zone, Minimum Inhibitory Concentrations (MIC), and IC50 methods. Essential oils exhibited antimicrobial activity against the tested strains. Results are comparable to the antibiotic Ampicillin, used as a positive control. Results (Table 1) show that essential oil inhibited the growth of bacterial strains. Depending on the susceptibility of the tested bacteria, it produced an inhibition zone varying from 6.2 to 28.4 mm for Gram positive bacteria and from 4.2 to 8.2 mm for Gram negative. Among Gram positive bacteria, highest inhibitory zone was observed against B. subtilis (28.4 mm) followed by S. aureus (24 mm) and E. cloacae (22.6 mm). Among Gram negative, highest inhibitory zone was observed against P. aeruginosa (8 mm). The inhibition zone for ampicillin (10 μg/disc), which was used as positive controls for bacteria, ranged from 20 to 26 mm.
Table 1.
Antibacterial activity of R. communis essential oil, using agar disc diffusion, IC50 and minimal inhibition concentration (MIC)
| Strains | DDa | IC50b | MICc | DDd |
|---|---|---|---|---|
| Bacterial strains Gram (+) | ||||
|
Bacillus subtilis |
28.4 ± 0.8 |
480 ± 1 |
>190.00 |
26 ± 0.6 |
|
Staphylococcus aureus 1327 |
24.0 ± 1.0 |
290 ± 5 |
>150.00 |
20 ± 0.5 |
|
Enterobacter cloacae |
22.6 ± 0.7 |
390.5 ± 19 |
>150.00 |
21 ± 1.4 |
|
Staphylococcus epidermidis |
16.6 ± 0.7 |
210 ± 5 |
>120.00 |
22 ± 0.5 |
|
Enterococcus faecalis |
15.2 ± 1.0 |
290 ± 10 |
>180.00 |
25 ± 1.0 |
|
Staphylococcus aureus 25923 |
14.0 ± 1.0 |
500 ± 20 |
>150.00 |
24 ± 0.5 |
|
Micrococcus luteus |
8.0 ± 0.5 |
230 ± 5 |
>140.00 |
20 ± 1.5 |
|
Bacillus cereus |
6.1 ± 0.9 |
300 ± 10 |
>130.00 |
21 ± 1.0 |
| Bacterial strains Gram (-) | ||||
|
Pseudomonas aeruginosa 27853 |
8.2 ± 0.8 |
530 ± 10 |
>270.00 |
20 ± 1.0 |
|
Klebsiella pneumoniae WHO24 |
6.2 ± 0.4 |
652 ± 8 |
>320.00 |
21 ± 0.9 |
|
Escherchia coli 25922 |
4.2 ± 0.9 |
430 ± 5 |
>240.00 |
22 ± 0.8 |
| Salmonella | 4.2 ± 0.6 | 590 ± 10 | >250.00 | 29 ± 1.0 |
Results are means of three different experiments.
aDD: Disc Diameter of inhibition (halo size) in millimeters, Essentiel.oil 100 μg/disc.
bMIC: minimum inhibitory concentration (in micrograms per milliliter).
cIC50: 50% inhibition concentration (in micrograms per milliliter).
dDD: Disc Diameter of inhibition zone of Ampicillin (10 μg/disc), was used as positive controls for bacteria.
For the fungi strains (Table 2), the disc diameter zones of inhibition ranged from 4.2-10.2 mm with a maximal inhibition zone obtained for P. digitatum.
Table 2.
Antifungal activity of R. communis essential oil using agar disc diffusion, IC50 and minimal inhibition concentration methods (MIC)
| Strains | DDa | IC50b | MICc | DDd |
|---|---|---|---|---|
| Fungal strains | ||||
|
Penicillium digitatum |
10.2 ± 0.2 |
350 ± 20 |
>140.00 |
21 ± 0.9 |
|
Fusarium solani |
08.2 ± 0.5 |
270 ± 30 |
>190.00 |
28 ± 0.6 |
|
Botrytis cinerea |
04.2 ± 0.6 |
590 ± 10 |
>250.00 |
29 ± 1.0 |
| Aspergillus niger | NA | NA | NA | 30 ± 0.5 |
Results are means of three different experiments.
aDD: Disc Diameter of inhibition (halo size) in millimeters, Essentiel.oil 100 μg/disc.
bMIC: minimum inhibitory concentration (in micrograms per milliliter).
cIC50: 50% inhibition concentration (in micrograms per milliliter).
dDD: Cycloheximide (10 μg/disc), was used as positive controls for fungi.
NA: no active.
The MIC and IC50 values of R. communis essential oil on bacteria ranged from 120 μg/ml to 300 μg/ml, and from 210 μg/ml to 870 μg/ml, respectively. Whereas the MIC and IC50 values on fungi ranged from 140 μg/ml to 250 μg/ml and from 350 μg/ml to 590 μg/ml.
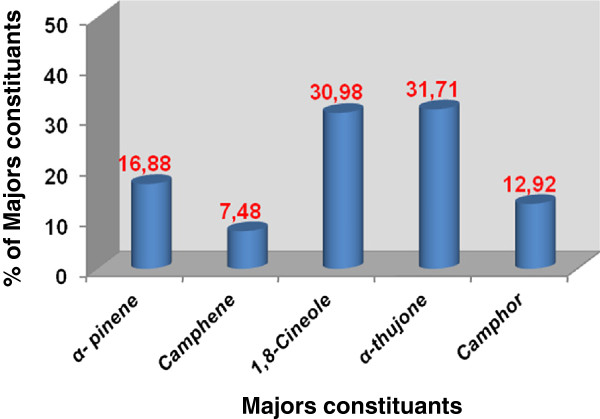
The antimicrobial activity of R. communis essential oil is strictly connected to their chemical compositions [30]. As it was reported previously (Figure 2) [20], the GC-MS analysis of R. communis essential oil using HP-5MS capillary column led the identification of five compounds accounting for 99.97% of the oil with a yield of 0.32%. The composition was predominantly by α-thujone and 1,8-cineole with equivalent contents of 31.71 and 30.98%, respectively, followed by α-pinene (16.88%), camphor (12.98%) and camphene (7.48%). Our results show that the variation in quantities of the main components e.g. camphor and 1,8-cineole, might be responsible for the different antimicrobial activity. Camphor as well as 1,8-cineole was revealed to inhibit the growth of bacteria and fungi [31,32]. Therefore, the detected antimicrobial properties of this essential oil could be due to the relatively high concentration of α-pinene (16.88%), which is believed to actively inhibit the growth of microorganisms [33]. The intense antimicrobial properties of essential oils from the aerial parts of R. communis was suspected to be associated with their high contents of oxygenated monoterpene (75.61%) appeared more active against the tested Gram positive than Gram negative bacteria. This result was in agreement with many studies realized on other plant species like E. robusta, E. alba, E. camadulensis, E. citriodora, E. globulus, E. saligna[34]. The effectiveness of this essential oil with containing a camphor at 12.98% against S. aureus 1327 (24 mm) and S. aureus 25923 (14 mm) are in agreement with those reported in the literature for other essential oil rich in camphor that showed a very strong action versus S. aureus[35]. Artemisia oils rich in camphor and 1,8-cineole were previously demonstrated to have potent antimicrobial activities in vitro[36].
Figure 2.
Chemical composition of R. communis essential oil.
Generally, Gram positive bacteria were more susceptible than Gram negative bacteria. B. subtilis and S. aureus were the most sensitive. While, P. aeruginosa, Salmonella and E. coli were the most resistant strain tested against this essential oil. Our results are in good agreement with the findings of Cantore et al. (2004) [37] who reported that Gram positive bacteria are more sensitive to plant essential oils than Gram negative bacteria, especially E. coli. The resistance of Gram negative bacteria against essential oils has been attributed to the presence of a hydrophilic outer membrane containing a hydrophilic polysaccharide chain, which acts as a barrier hydrophobic essential oil [38].
Essential oils always represent a complex mixture of different chemical components. Thus, it is very difficult to attribute the antibacterial effect of the total oil to a few active principles. In general, it was also possible that the compounds in lower percentage might be involved in some type of synergism with the active compound [30].
Cytotoxicity assays
The effect of different concentrations of R. communis essential oil on HeLa cell survival was studied. The results summarized in Table 3, show that the R. communis essential oil exhibited a moderate inhibitory effect on the cervical cancer line examined. At a concentration of 3 mg/ml, essential oil destructed HeLa cells by about 30%, however at at a concentration of 4 mg/ml, almost all HeLa cells were destructed. Cytotoxicity was expressed as the concentration of oil inhibiting cell growth by 50% (IC50). The IC50 value of R. communis essential oil was evaluated to 2.63 mg/ml. Therefore, doses under this concentration were used for biological antioxidant activity investigation. R. communis essential oil was able to exert antiproliferative activity against Hela cell line, this result suggests a specific mechanism of action interfering with abnormal proliferation.
Table 3.
Cytotoxic effects of R. communis essential oil on HeLa cell line by MTT assay
| Essential oil (μg/ml) | % cell viability |
|---|---|
| 0 |
100.00 |
| 125 |
98.90 |
| 500 |
95.77 |
| 1000 |
89.72 |
| 1500 |
84.70 |
| 2000 |
88.80 |
| 2200 |
70.00 |
| 2360 |
60.00 |
| 2600 |
46.00 |
| 3000 |
28.27 |
| 3500 |
01.53 |
| 4000 | 0.210 |
Results are presented as viability ratio. Values were expressed as the mean of at least three independent experiments.
Our study confirmed the results reported previously by Kadri et al. [20], who indicated that the antioxidant activity of R. communis essential oil was efficient when concentrations are less than 0.4 mg/ml. The cytotoxicity of this essential oil was attributed to the presence of α-pinene (16.88%) [39], and to the synergetic effect between minor and major compounds [39].
Conclusion
In conclusion, essential oil of R. communis showed significant antimicrobial and antiproliferative activities. α-thujone, 1,8-cineole, α-pinene, Camphor and camphene were common in the oil as five major compounds. The results suggest that R. communis essential oils possess some compounds with antimicrobial and antiproliferative properties, which can be used as antimicrobial agents in new drugs for treatment of infectious diseases. It is quite difficult to attribute the antimicrobial and the cytotoxic effects of an essential oil to one or a few active principles, because extracts always contain a mixture of different chemical compounds. In addition to the major components, also minor components may make a significant contribution to the biological activity of extracts. Following the results above, we could infer that the antimicrobial and the cytotoxic effects of R. communis essential oil is the synergistic effect of their compositions.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
ZZ, IBC, RBM and AB carried out the experimental part such as extraction, antibacterial, antifungal and cytotoxicity assays. ZZ contribute to the analysis of the results. NG and AK supervised the work and corrected the manuscript. Authors read and approved the final manuscript.
Contributor Information
Zied Zarai, Email: zaraizied@hotmail.fr.
Ines Ben Chobba, Email: inesbenchobba@yahoo.fr.
Riadh Ben Mansour, Email: riadhbm@yahoo.fr.
Ahmed Békir, Email: ahmed.bekir@voila.fr.
Néji Gharsallah, Email: Neji.Gharsallah@fss.rnu.tn.
Adel Kadri, Email: lukadel@yahoo.fr.
References
- Heath HB. Source Book of Flavours. Westport: Avi; 1981. pp. 1–890. [Google Scholar]
- Alim A, Goze I, Cetin A, Atas AD, Vural N, Donmez E. Antimicrobial activity of the essential oil of Cyclotrichium niveum (Boiss.) Manden. Et Scheng. Afr J Microbiol Res. 2009;3(8):422–425. [Google Scholar]
- Watson RR, Preedy VR. Botanical Medicine in Clinical Practice. ND: Cabi Publishing; 2008. pp. 165–166. [Google Scholar]
- Edris AE. Pharmaceutical and therapeutic potential of essential oils and their individual volatile constituents: a review. Phytoth Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- Burt S. Essential oils: their antimicrobial properties and potential application in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Celiktas OY, Kocabas EEH, Bedir E, Sukan FV, Ozek T, Baser KHC. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2006;100:553–559. [Google Scholar]
- Sokmen M, Serkedjieva J, Daferera D, Gulluce M, Polissiou M, Tepe B, Akpulat HA, Sokmen A. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J Agric Food Chem. 2004;52:3309–3312. doi: 10.1021/jf049859g. [DOI] [PubMed] [Google Scholar]
- Sokovic M, Van Griensven LJLD. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom, Agaricus bisporus. Eur J Plant Pathol. 2006;116:211–224. doi: 10.1007/s10658-006-9053-0. [DOI] [Google Scholar]
- Hussain AI, Anwar F, Sherazi STH, Przybylski R. Chemical composition. Antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008;108:986–995. doi: 10.1016/j.foodchem.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Bozin B, Mimica-Dukic N, Simin N, Anackov G. Characterization of the volatile composition of essential oils of some lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J Agric Food Chem. 2006;54:1822–1828. doi: 10.1021/jf051922u. [DOI] [PubMed] [Google Scholar]
- Kelen M, Tepe B. Chemical composition, antioxidant and antimicrobial properties of the essential oils of three Salvia species from Turkish flora. Bior Technol. 2008;99:096–4104. doi: 10.1016/j.biortech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ferraz A. Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from Ricinus communis. Pharmacol Biochem Be. 1999;63:367–375. doi: 10.1016/S0091-3057(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BA. Indian Medicinal Plants. 3. 1991. pp. 2274–2277. [Google Scholar]
- Dhar ML, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants for biological activity. Part I. Indian J Exp Biol. 1998;6:232–247. [PubMed] [Google Scholar]
- Capasso F, Mascolo N, Izzo AA, Gaginella TS. Dissociation of castor oil induced diarrhea and intestinal mucosal injury in rat: effect of NG-nitro-L- arginine methyl ester. B J Pharmacol. 1994;113:1127–1130. doi: 10.1111/j.1476-5381.1994.tb17113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguet TT. New world material medica in Spanish renaissance medicine from scholarly reception to practical impact. Med Hist. 2001;45(3):359–376. [PMC free article] [PubMed] [Google Scholar]
- Gibbs S, Harvey I, Sterling J, Stark R. Local treatments for cutaneous warts systemic review. BMJ. 2002;352(7362):461–464. [PMC free article] [PubMed] [Google Scholar]
- Wilcox ML, Bodeker G. Traditional herbal medicines for malaria. BMJ. 2004;329(7475):1156–1159. doi: 10.1136/bmj.329.7475.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavarasan R, Mallika M, Ventakaraman S. Anti-inflammatory and free radical scavenging activity of Ricinus communis root extract. J Ethnopharmacol. 2006;103(3):478–480. doi: 10.1016/j.jep.2005.07.029. [DOI] [PubMed] [Google Scholar]
- Kadri A, Gharsallah N, Damak M, Gdoura R. Chemical composition and in vitro antioxidant properties of essential oil of Ricinus communis L. J Med Plant Res. 2011;5(8):1466–1470. [Google Scholar]
- Berghe VA, Vlietinck AJ. Screening methods for antibacterial and antiviral agents from higher plants. Method Plant Biochem. 1991;6:47–68. [Google Scholar]
- Wade D, Silveira A, Rollins-Smith L, Bergman T, Silberring J, Lankinen H. Hematological and antifungal properties of temporin A and a cecropin A-temporin A hybrid. Acta Biochim Pol. 2001;48:1185–1189. [PubMed] [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards) Proceedings of the ninth International Supplement. Wayne: NCCLS; 1999. Performance standard for antimicrobial disk susceptibility testing; p. PA100-S9. [Google Scholar]
- May J, Chan CH, King A, Williams L, French GL. Time-kill studies of tea tree oils on clinical solates. J Antimicrob Chemoter. 2000;45:639–643. doi: 10.1093/jac/45.5.639. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Proenca AC, Serralheiro CMLM, Araujo MEM. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J Ethnopharmacol. 2006;108:31–37. doi: 10.1016/j.jep.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Saidana D, Mahjoub MA, Boussaada O, Chriaa J, Cheraif I, Daami-Remadi M, Mighri Z, Helal AN. Chemical composition and antimicrobial activity of volatile compounds of Tamarix boveana (Tamaricaceae) Microbiol Res. 2008;163:445–455. doi: 10.1016/j.micres.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif I, Daami-Remadi M, Helal AN, Mighri Z. Chemical composition and antimicrobial activity of volatile components from capitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbiol Res. 2008;163:87–95. doi: 10.1016/j.micres.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Omar-Hamza JM, Carolien JP, Van Den Bout-van Den Beukel, MeckyMatee IN, Paul-Verweij JAME. Antifungal activity of some Tanzanian plants used traditionally for the treatment of fungal infections. J Ethnopharmacol. 2006;108:124–132. doi: 10.1016/j.jep.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–29. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- De Vincenzi M, Mancini E, Dessi MR. Monographs on botanical avouring substances used in foods. Part V. Fitoterapia. 1996;67:241–251. [Google Scholar]
- Tabanca N, Kirimer N, Demirci B, Demirci F, Baser KHC. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J Agric Food Chem. 2001;49:4300–4305. doi: 10.1021/jf0105034. [DOI] [PubMed] [Google Scholar]
- Dorman HJD, Deans SG. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J Appl Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- Hussain AI, Anwar F, Shahid M, Ashraf M, Przybylski R. Chemical composition, anticoidant and antimicrobial activities of essential oil of spearmint (Mentha spicata L.) from Pakistan. J Ess Oil Res. 2010;22:78–84. doi: 10.1080/10412905.2010.9700269. [DOI] [Google Scholar]
- Charchari S, Dahoun A, Bachi F, Benslimani A. Antimicrobial activity in vitro of essential oils of Artemisia herba-alba Asso and Artemisia judaica L. from Algeria. Riv Ital EPPOS. 1996;18:3–8. [Google Scholar]
- Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, Totte J, Pieters L, Vlietinck AJ. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J Ethnopharmacol. 2002;79:213–220. doi: 10.1016/S0378-8741(01)00384-1. [DOI] [PubMed] [Google Scholar]
- Cantore PL, Iacobellis NS, Marco AD, Capasso F, Senatore F. Antioxidant activity of Coriandrum sativum L. and Foeniculum vulgare Miller Var. vulgare (Miller) essential oils. J Agric Food Chem. 2004;52:7862–7866. doi: 10.1021/jf0493122. [DOI] [PubMed] [Google Scholar]
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish Artemisia Species. J Agric Food Chem. 2005;53:1408–1416. doi: 10.1021/jf048429n. [DOI] [PubMed] [Google Scholar]
- Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96(1–2):151–158. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]