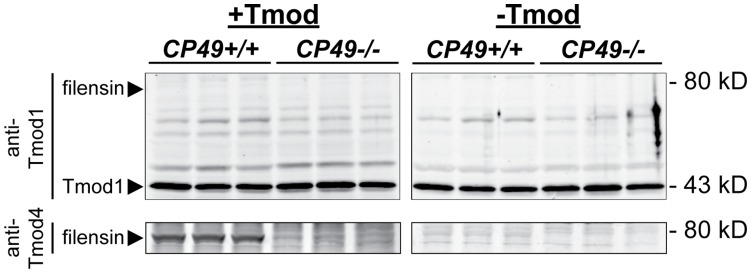
Figure 7. Tmod1 does not bind filensin in a blot overlay assay.
CP49+/+ and CP49−/− mouse lens extracts were separated by SDS-PAGE and transferred to nitrocellulose. Blots were pre-incubated in the presence (+Tmod) or absence (-Tmod) of 2 µg/ml Tmod1 (upper panels) or 2 µg/ml Tmod4 (lower panels). Tmod-labeled blots were then probed with antibodies recognizing either Tmod1 or Tmod4, as indicated, to reveal endogenous Tmod1 (∼43 kD) and recombinant Tmod4 (but not Tmod1) bound to mouse filensin at ∼80 kD (arrowheads). The Tmod4-binding band at ∼80 kD was dramatically reduced in CP49−/− extracts, confirming its identity as filensin, since CP49−/− lenses contain only 1/5th of wild-type filensin levels (Fig. 1 and [18], [20], [41]). The ∼43 kD band in the upper panels corresponds to endogenous Tmod1 labeled by the anti-Tmod1 antibody.