Abstract
Effortful control (EC), or the trait-like capacity to regulate dominant responses, has important implications for children’s development. Although genetic factors and parenting likely influence EC, few studies have examined whether they interact to predict its development. The current study examined whether the DRD4 exon III variable number tandem repeat polymorphism moderated the relationship between parenting and children’s EC. A total of 382 three-year-olds and primary caregivers completed behavioural tasks assessing children’s EC and parenting. Children’s DRD4 genotypes moderated the relationship between parenting and EC: children with at least one 7-repeat allele displayed lower EC in the context of negative parenting than children without this allele. These findings suggest opportunities for modifying early risk for low EC.
Keywords: effortful control, dopamine D4 receptor, parenting
The temperament trait effortful control (EC) has been defined as “the ability to suppress a dominant response in order to perform a subdominant response” (Rothbart, Ellis, Rueda & Posner, 2003, p. 1114). EC is a broad construct comprised of several components including attentional and inhibitory control, and a factor analysis of parent-reported temperament scales by Rothbart and colleagues yielded an EC factor defined by these regulatory facets as well as low intensity pleasure and perceptual sensitivity (Rothbart, Ahadi, Hershey & Fisher, 2001). EC is thought to incorporate the focusing and shifting of attention, the suppression of inappropriate responses in accordance with social cues, and the capacity to plan for the future and modify behavior accordingly (Rothbart, 2007). Importantly, EC not only facilitates the inhibition of dominant responses but also activates non-dominant responses (Eisenberg, Fabes & Murphy, 1995). For example, while EC is needed to inhibit behavior in situations where doing so affords the possibility of attaining a more desirable reward in the future, EC also plays a role in motivating engagement in activities with potential benefits, despite experiencing fear, anxiety or boredom (Carver, Johnson, & Joormann, 2008).
EC typically emerges near the end of the first year of life, demonstrating particularly rapid development in the preschool years, and continues to mature throughout early childhood and into adolescence (Kochanska, Murray & Harlan, 2000). Despite this ongoing growth, the rank order of EC remains relatively stable throughout toddlerhood and into the early school years (Kochanska & Knaack, 2003). EC has been linked both concurrently and prospectively to an array of important outcomes, such as children’s development of prosocial emotions (Eisenberg, Smith, Sadovsky & Spinrad, 2004), conscience (Kochanska & Askan, 2006) and social adjustment (Eisenberg et al., 2004). EC has also been implicated in child psychopathology risk, with multiple studies linking deficits in EC to both externalizing and internalizing disorders (e.g., Eisenberg et al., 2009), although Carver and colleagues (2008) recently argued that the role of EC in psychopathology cannot be precisely delineated without considering its moderating effect on other temperament traits.
With respect to factors that likely shape children’s emerging EC, evidence for the important role of genetic factors comes from twin studies, which show heritability estimates up to 79% (Lemery-Chalfant, Doelger & Goldsmith, 2008). While less is known about the effects of specific genetic polymorphisms, a consideration of the neurophysiological underpinnings of EC suggests potential genetic candidates. Rothbart and colleagues (1994) proposed that EC is supported by a network of brain regions called the executive attention network. As such, individual differences in EC are often defined as variations in the efficiency of the executive attention network (Posner & Fan, 2005). This network, which includes the anterior cingulate cortex (ACC) and the lateral prefrontal cortex (PFC) as its neurobiological substrates, is thought to be involved in executive attention tasks such as the regulation of sensory and motor regions, and the resolution of conflict between different brain regions and competing stimuli (Rueda, Posner, & Rothbart, 2004). Posner and Fan (2005) argue that the executive attention network is modulated by the neurotransmitter dopamine. Consistent with this proposal, brain areas associated with executive attention receive strong projections from the ventral tegmental area, a dopamine-rich region. In addition, the cingulate is considered particularly rich in dopamine innervations (Berger, Gaspar, Verney, 1991) and receptors, especially the dopamine D4 receptor, are densely populated in this region (Boy et al., 1998). Furthermore, injection of dopamine antagonists impairs performance on tasks requiring executive attention, and dopamine depletion in the dorsal lateral prefrontal cortex impairs performance on executive attention tasks (Nieoullon, 2002). Thus, it is clear that dopamine plays an important role in the executive attention network and in EC, suggesting the likelihood that specific genetic polymorphisms that influence dopaminergic neurotransmission might also shape EC.
Consistent with the role of dopaminergic neurotransmission in EC, the dopamine D4 receptor (DRD4) gene (Gene ID: 1815) has been most consistently related to measures of attention, and its polymorphic variants are thought to have direct biochemical implications for attention by promoting synchronized firing of neuronal networks (Deth, Kuznetsova, Waly, 2004). Found on chromosome 11p15, DRD4 is highly polymorphic (Wang et al., 2004). A variable number tandem repeat (VNTR) located in the third exon of the gene codes for the third intracellular loop of the resulting receptor protein. The number of tandem repeats varies across individuals from two to eleven repeats, with 2-, 4- and 7- repeats being the most frequent variants in Caucasians. The 7-repeat variant exhibits decreased signal transduction efficiency relative to the 4-repeat variant (Asghari et al., 1995), and may also have decreased RNA stability or translational efficiency (Schoots & Van Tol, 2003). Furthermore, there are significant differences between receptor variants in folding efficiency when shaping the final protein product, such that the mRNA transcript of the DRD4 2-repeat allele folds more quickly into a protein product than the transcripts of longer alleles, thus increasing DRD4 signaling (van Craenenbroeck et al., 2005). Cumulatively, these effects are likely to have a significant impact on the signaling and functioning of neural circuits involved in EC.
In addition to the observed biochemical effects of the various DRD4 exon III VNTR variants, behavioral associations that further support the role of these variants in EC have been reported. First, several meta-analyses suggest that the 7-repeat allele is associated with symptoms of attention deficit hyperactivity disorder (e.g., Faraone, Doyle, Mick, & Biederman, 2001). The 7-repeat allele is also associated with decreased attention in non-clinical samples of infants and preschoolers (Auerbach, Benjamin, Faroy, Geller, & Ebstein, 2001; Schmidt, Fox, Perez-Edgar, Hu, & Hamer, 2001), further suggesting that this variant influences attentional processes, an important facet of EC. Similarly, in a study of college students, individuals with the 7-repeat allele demonstrated poorer inhibitory control (Congdon, Lesch, & Canli, 2008). However, contradictory findings have also been reported; for example, in a sample of adults, Kramer and colleagues (2009) found that the 7-repeat allele was related to increased cognitive ability and greater inhibitory control. Additionally, Fossella and colleagues (2002) reported that the 4-repeat allele, rather than the 7-repeat, was related to deficits in executive attention in a sample of adults. Thus, despite evidence suggesting that DRD4 exon III VNTR polymorphic variants are related to EC, the exact nature of the relationship remains unclear.
In addition to significant genetic influences, a large literature shows that EC is also shaped by early experience, especially parenting (Karremann, van Tuijl, van Aken & Dekovic, 2006). For example, Karremann, van Tuijl, van Aken and Dekovic (2008) found that parent self-reported responsiveness and positive control, a construct that includes limit-setting and providing structure, were positively associated with child EC. Similarly, Lengua, Honorado and Bush (2007) found that observed maternal limit-setting, scaffolding, and respect for child autonomy were related to increases in observed EC over a six-month period. Maternal self-reports of sensitivity, acceptance and support were positively related to their children’s observed EC both concurrently and eleven months later (Kochanska et al., 2000). Self-reported and observer-rated maternal expressions of positive emotions were positively associated with children’s regulation, while maternal expressions of negative emotion were negatively associated with EC (Eisenberg, Gershoff et al., 2001). Kochanska and Knaack (2003) found that observed maternal power assertion, including the use of physical discipline, was negatively associated with child EC. Positive and negative parenting practices potentially influence EC through a number of mechanisms (Blair, 2002; Eisenberg et al., 2005; Grusec & Goodnow, 1994; Hoffman, 2000; Valiente et al., 2006). For example, negative or hostile parenting may increase children’s levels of negative emotionality, which may interfere with their ability to engage in complex cognitive processes, such as those that underlie EC (Blair, 2002). In contrast, children in a positive parent-child relationship may be more motivated to internalize parental directions, a process necessary for conceptualizing alternative non-dominant responses (Grusec & Goodnow, 1994). Also, parenting practices may be a reflection of parents’ own EC, suggesting that a passive gene-environment correlation may exist (Rutter, 1997) such that parenting and child EC are influenced by the same genetic variants.
However, it is likely that children differ widely in their susceptibility to the effects of both positive and negative parenting behaviors (Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2007; Belsky & Pluess, 2009; Ellis & Boyce, 2008; Rutter, Moffitt & Caspi, 2006). Studies of gene-environment interactions (e.g., Rutter et al., 2006) posit a genetic vulnerability model (or diathesis-stress model; Monroe & Simons, 1991) in which individuals possessing particular genetic variants experience enhanced risk for maladaptive outcomes in the presence of negative contextual influences, including poor parenting. For example, maternal unresolved loss or trauma was associated with infant disorganized attachment, but only in those children with the 7-repeat allele (Van Ijzendoorn & Bakermans-Kranenburg, 2006; see also Bakermans-Kranenburg, van Ijzendoorn, Pijlman, Mesman & Juffer, 2008) Alternatively, according to Belsky and colleagues (2007) and others (Ellis & Boyce, 2008; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & Van Ijzendoorn, 2011), some genetic variants may not simply confer risk or resilience, but instead confer a general sensitivity to contextual factors, which can result in either positive or negative outcomes depending on the specific context. A growing literature supports this theory of differential susceptibility (e.g., Bakermans-Kranenburg, & Van Ijzendoorn, 2011). For example, maternal sensitivity assessed at 10 months was associated in a bivalent manner with child externalizing problems at 39 months, but only in those children with the 7-repeat allele of the DRD4 receptor gene (Bakermans-Kranenburg, & van Ijzendoorn, 2006); children with the 7-repeat allele showed either relatively low or high levels of externalizing problems, depending on whether mothers were high or low in sensitivity, respectively (however, see Propper, Willoughby, Halpern, Carbone, & Cox, 2007 for contradictory results). Thus, parenting may interact with genetic polymorphisms in the DRD4 exon III VNTR region following a pattern consistent with either diathesis-stress or differential susceptibility models to influence child behaviors and outcomes.
To date, however, there is little research comparing different models of gene-environment interplay in predicting EC. Sheese, Voelker, Rothbart and Posner (2007) examined the interaction between DRD4 exon III VNTR polymorphisms and parenting in predicting sensation seeking and EC. Results indicated that lower quality parenting resulted in greater sensation seeking in children with the 7-repeat allele than those without the 7-repeat allele. They failed to find a main or moderated effect of allelic variation in DRD4 in predicting EC; however, the small sample size of this research (N = 45) limited the power of this study to detect genetic influences on EC. Also, participants in this study were 18 to 21 months of age. Since EC does not crystallize until around 3 to 4 years of age, it is possible that estimates of EC in younger populations are subject to greater measurement error than those obtained in older children. Furthermore, researchers in this study aggregated across all parenting variables, positive and negative, to create a single index of parenting quality. However, aggregating across these variables may obscure potentially important relationships if positive and negative parenting have differential effects of on children’s self-regulation (e.g., Karreman et al., 2006;).
To further explore whether and how genetic and contextual factors shape EC, and to address the limitations of previous studies, we examined the role of parenting and the DRD4 7-repeat allele in predicting childhood EC. We focused primarily on the inhibitory control aspect of EC, which is defined as the ability to inhibit impulsive behavior in accordance with social or contextual motivation (Rothbart, Ahadi, & Hershey, 1994). Inhibitory control is particularly relevant to an array of critical outcomes including the development of conscience, theory of mind and attention-deficit/hyperactivity disorder (Carlson & Moses, 2001; Kochanska& Askan, 2006; Schachar, Mota, Logan, Tannock, & Klim, 2000). Moreover, as previously described, this facet of EC may show the strongest association with DRD4 polymorphic variants (e.g., Congdon et al., 2008) although contradictory findings have also been reported (e.g., Kramer et al., 2009). Given the important role of parenting in the development of EC, we expected that both positive and negative parenting factors would be associated with children’s EC. However, in light of preliminary evidence that the DRD4 7-repeat allele sensitizes children to parenting influences, we expected that the relationship between parenting variables and EC would be moderated by DRD4 exon III VNTR genotype, such that the associations between negative parenting, and perhaps positive parenting, and children’s EC would be more pronounced in children with the DRD4 7-repeat allele. Given the lack of a relevant literature to draw upon, we did not develop a specific prediction regarding whether this interaction would be consistent with diathesis-stress or differential susceptibility models.
Method
Participants
The sample in this report consisted of 382 children (202 males) with complete data on the genetic, EC, and parenting measures. They came from a larger sample of 559 children and their parents from a suburban area. The mean age of the children was 42.20 months (SD = 3.12). The mean age of mothers was 36.08 years (SD = 4.46) and fathers were 38.38 years old (SD = 5.32). The majority of children (61.51%) had a least one older sibling. Potential participants were identified via a commercial mailing list and initially contacted by the Stony Brook University Center for Survey Research. Eligible families had a child between three and four years of age, with no significant medical conditions or developmental disabilities, and at least one English-speaking, biological parent. Most of the participants came from middle class families, as measured by Hollingshead’s Four Factor Index of Social Status (Hollingshead, 1975; M = 44.78; SD = 10.94). The vast majority (96.39%) of children came from two-parent homes, and 50.60% of the mothers worked outside the home part- or full-time. All caregivers in the present sample were reported to be the child’s biological parent, although formal paternity testing was not conducted. Rates of parental psychopathology were consistent with those expected in a community sample such as this (Olino, Klein, Dyson, Rose & Durbin, 2010). Children were administered the Peabody Picture Vocabulary Test (M = 103.13, SD = 13.67) (PPVT; Dunn & Dunn, 1997) to screen for gross cognitive impairment.
Procedures and Measures
Genotyping
To type the DRD4 exon III VNTR polymorphisms, genomic DNA was extracted using Qiagen DNA MicroKit® (Qiagen Valencia, CA, USA) according to manufacturer’s protocols. Genomic DNA was successfully extracted for all 476 children who provided buccal swabs for analysis. To reduce the possibility of population stratification, all non-White children or those of unknown ethnicity in the original sample were excluded from the current study (N = 63), leaving a sample of 413 children. The 48-base pair VNTR located in the third exon of the DRD4 gene was amplified using a 25 µl reaction containing 25 ng of genomic DNA template with forward primer 5’-CGCGACTACGTGGTCTACTCG-3’ and reverse primer 5’-AGGACCCTCATGGCCTTG-3’, and 1 U of NovaTaq polymerase (Novagen, Gibbstown, New Jersey, USA). The reaction also included 2 mM each of dATP, dCTP and dTTP, 1mM each of dGTP, dITP, with 10% DMSO and 1X PCR amplification buffer (20 mmol/l Tris-HCL pH 8.4, 50 mmol/L KCL). PCR amplification was carried out in a GeneAmp PCR System 9700 (ABI Biosystems, Foster City, California, USA). Following an initial denaturation at 95°C for 5 minutes, thirty cycles of amplification were run with each cycle consisting of denaturation at 95°C for 20 sec, annealing at 54°C for 20 sec, and extension at 72°C for 40 sec, ending with a final extension step of 5 min at 72°C. The PCR amplicons were then resolved on a 2% agarose gel, stained with ethidium bromide (Sigma, Oakville, Ontario, Canada) and documented on the Bio-Rad 1300 Gel documentation system (Mississauga, Ontario, Canada). Product sizes were determined against a 100 bp molecular weight standard (Invitrogen, Carlsbad, California, USA).
The DRD4 VNTR polymorphism, like other VNTRs, has many possible variants (Wang et al., 2004), ranging from 2- to 11-repeat copies reported in the literature to date. In our sample, the following genotypes were found: 2/2 (N = 12, 2.9%), 2/4 (N = 40, 9.7%), , 2/5 (N = 1 ,.2%), , 2/7 (N = 11, 2.7%), 3/3 (N = 5, 1.2%),3/4 (N = 10, 2.4%), 3/5 (N = 10, 2.4%), 3/7 (N = 1, .2%),4/4 (N = 176, 42.6%), 4/5 (N = 6, 1.5%), 4/6 (N = 1, .2%), 4/7 (N = 102, 24.7%) ,4/8 (N = 2, .5%) 5/7 (N =1, .2%), and 7/7 (N = 35, 8.5%). This genotype distribution is not consistent with Hardy-Weinberg equilibrium (Pearson X2(21) = 239.58, p < .05; Guo & Thompson, 1992), but is comparable to recently reported frequencies (Ding et al., 2002). All genotyping was performed by research technicians blind to other study data. To further ensure genotyping accuracy, a subset of children (N = 145) were genotyped again by an independent laboratory; all results were identical to those reported here. Consistent with the majority of published research (e.g., Faraone et al., 2001; Sheese et al., 2007), groups for data analysis were formed based on whether children had (N = 150) or did not have (N = 263) a 7-repeat allele.
Laboratory temperament assessment
During a two and a half hour laboratory visit, each child participated in a standardized set of 12 episodes drawn from the Laboratory Temperament Assessment Battery (Lab-TAB; Goldsmith, Reilly, Lemery, Longley, & Prescott, 1995). A female experimenter led each child through the tasks, which were video recorded for coding purposes. Two of the twelve tasks were used to assess EC and are described below; the other tasks in the battery will not be discussed further here. Due to the need to keep the structure of the visit consistent across participants and to the number of tasks that comprised the battery, we did not attempt counterbalancing of behavioral tasks; however, children were given short breaks between tasks to allow them to return to baseline and tasks designed to elicit similar responses were not conducted consecutively (e.g., the two EC tasks examined in this paper were separated by 8 tasks). EC data were obtained for 411 of the 413 Caucasian participants who provided DNA.
Tower of patience
A female experimenter and child took turns stacking large cardboard blocks to build a tower. The experimenter waited a series of increasing delays (5, 10, 15, 20, 30 s) before placing her block on the tower, thus forcing the child to wait increasingly longer periods of time before being given a turn. Two towers were built over the course of the task.
Snack delay
The experimenter placed a chocolate candy underneath a transparent cup, telling the child that (s)he must wait until the experimenter rang a bell before picking up the cup and eating the candy. The experimenter adhered to a series of delays of increasing length (5, 10, 20, 30s), forcing the child to wait longer each time to eat the candy.
As an index of EC, each task was coded for failures to wait (i.e., placing a block out of turn during the tower task, or eating the candy before the bell was rung during the snack task; see Carlson, 2005, Kochanska, Murray, Jacques, Koenig & Vandegeest, 1996, and Kochanska, & Knaack, 2003, for similar procedures). The number of failures to wait was counted for each delay. These values were then averaged across each delay and then averaged again across tasks to create an aggregate EC scale. The tasks were coded by four undergraduate research assistants who completed extensive training. Coders were blind to child DRD4 VNTR genotype and parenting measures. Raters had to reach at least 80% agreement with a “master” rater before coding independently. To examine interrater reliability, 8 of the videotapes were independently coded by a second rater (ICC = .98).
Parenting Assessment
Observed measures of parenting were collected for 384 of the 413 Causcasian participants who gave DNA. As two of these children did not have data on EC, the final sample was 382. Children and a primary caregiver (usually the biological mother, N = 374, 97.9%) participated in a 30-minute series of six standardized parent-child interaction tasks designed to elicit different parenting and child behaviors, based on the Teaching Tasks battery (Egeland et al., 1995). Again, due to the need to keep the assessment consistent across participants, and due to the number of tasks that comprised the battery, the interaction tasks, described below, were not counterbalanced.
In the first task, which lasted approximately five minutes, the parent and child read and discussed a short book. In the second task, the parent encouraged the child to name as many things with wheels as possible for a period of approximately four minutes. A third task, lasting approximately five minutes, required the parent and child to build large square blocks from a set of smaller blocks. During the fourth task, which lasted approximately three minutes, the parent helped the child match game pieces based on colour and shape. The fifth task, which was five minutes in length, called for the parent to assist the child in completing a maze by turning knobs on an Etch A Sketch™. In the final task, the parent presented the child with a small gift and the two spent approximately two minutes playing with the toy.
The interaction was videotaped and later coded using a global approach, where a single global rating was made for each parenting variable for each task based on all relevant behaviors in that episode. Ratings of positive and negative parenting behaviors were made by the same rater for each participant. A total of eight raters (one graduate student, three bachelors-level research assistants, and four undergraduate research assistants) contributed to coding. See Table 1 for a description of coding procedures. Interrater ICCs for supportive presence, instructional quality, positive affect, confidence, relationship quality, hostility, intrusiveness and negative affect were .85, .80, .66, .59, .79, .83, .70, and .73 respectively.
Table 1.
Parent-Child Interaction Coding Descriptions
| Scale | Cronbach’s Alpha |
Scale Type |
Description | Behavioural Examples | |
|---|---|---|---|---|---|
| Positive Parenting | .82 | ||||
| Supportive Presence | .88 | 5 pt Likert | parental positive regard and emotional support to child |
|
|
| Quality of Instruction | .67 | 5 pt Likert | the parent’s ability to clearly describe the task objectives and appropriately instruct the child to completion of these objectives |
|
|
| Confidence | .66 | 3 pt Likert | the degree to which the parent appeared sure of his/hers ability to work successfully with the child to achieve task objectives while maintaining appropriate child behavior |
|
|
| Positive Affectivity | .84 | 3 pt Likert | the frequency and intensity of the parent’s expression of positive emotion |
|
|
| Quality of Relationship | .86 | 5 pt Likert | the degree to which the dyad exhibited mutual or reciprocal engagement |
|
|
| Negative Parenting | .66 | ||||
| Intrusiveness | .61 | 5 pt Likert | the degree to which the parent interferes with the child’s needs, interests, or actual behaviors and failures to recognize the child’s effort to gain autonomy |
|
|
| Hostility | .76 | 5 pt Likert | the parent’s expression of anger, frustration, annoyance, discounting or rejecting of the child |
|
|
| Negative Affectivity | .77 | 3 pt Likert | the frequency and intensity of the parent’s expression of negative affect |
|
|
To reduce the number of scales for analyses, a principal components analysis of the parenting variables was conducted, which yielded a two-factor solution. The first factor, which accounted for 50.74% of the total variance, included loadings from supportive presence (.75), instructional quality (.73), confidence (.74), positive affect (.65) and quality of relationship (.72). This factor was named positive parenting. The second factor, which accounted for 13.36% of the total variance, included loadings from parent hostility (.85), intrusiveness (.64), and negative affect (.86). This factor was called negative parenting. Two aggregates were formed by averaging the standardized scores for the ratings that loaded on each factor, one representing positive parenting and the second representing negative parenting. The positive and negative parenting aggregates were significantly correlated (r = −.56, p < .001). Because negative parenting values were positively skewed, a square root transformation was applied and transformed values were used in all analyses.
Results
Table 2 shows descriptive statistics for the study and demographic variables for the two child DRD4 exon III VNTR genotype groups. Groups based on genotype did not differ in total failures to wait, indicating no direct association between this gene and EC. Similarly, groups based on genotype did not differ on the positive parenting aggregate or the negative parenting aggregate, indicating no association between children’s DRD4 genotypes and the parenting they received. Consistent with previous findings indicating sex differences in EC (e.g., Else-Quest, Hyde, Goldsmith, & Van Hulle, 2006), boys (M = .19, SD = .17) demonstrated more failures to wait (averaged across all trials) than girls (M = .13, SD = .13; t(461) = 4.48, p < .001). Since PPVT scores (r = −.24, p < .001) and child age (r = −.12, p < .05) were also associated with failures to wait, all these variables were included as covariates in analyses, although findings were similar without these covariates in models.
Table 2.
Demographic and Study Variables by Child DRD4 Exon III VNTR Genotype
| Child DRD4 Exon III VNTR Genotype | ||||||
|---|---|---|---|---|---|---|
| 7-Repeat Absent (N=243) | 7-Repeat Present (N=139) | |||||
| Variable | M | SD | N | M | SD | N |
| EC | .15 | .14 | .16 | .16 | ||
| Child Sex (Male) | 129 (53%) | 73(52%) | ||||
| PPVT | 103.10 | 13.40 | 103.67 | 13.40 | ||
| SES | 45.39 | 10.91 | 44.08 | 10.49 | ||
| Positive Parenting | 0.00 | 0.34 | 0.01 | 0.34 | ||
| Negative Parenting | 0.00 | 0.09 | 0.01 | 0.10 | ||
Note: EC = Effortful Control, PPVT = Peabody Picture Vocabulary Test, SES = socioeconomic status, as indexed by Hollingshead’s Four Factor Index of Social Status (Hollingshead, 1975).
Consistent with previous work showing associations between parenting and EC (Eisenberg et al., 2005; Kochanska et al., 2000; Lengua et al., 2007; Valiente et al., 2006), children receiving higher levels of positive parenting demonstrated fewer failures to wait (r = −.14, p < .001). In contrast, children receiving higher levels of negative parenting demonstrated more failures to wait (r = .23, p < .001). We examined whether these associations between parenting and EC were moderated by children’s DRD4 exon III VNTR genotypes using multiple regression (Aiken & West, 1991). All predictor variables were centered as appropriate. After entering PPVT scores, child sex, and child age as covariates, positive parenting, negative parenting and child DRD4 genotype were entered followed by the products of the two parenting variables with DRD4 genotype (i.e., positive parenting × DRD4 genotype and negative parenting × DRD4 genotype). Neither the main effect of positive parenting, nor the interaction between positive parenting and DRD4, was significant in the full model (Table 3). However, the interaction term between negative parenting and DRD4 genotype was significant, indicating that the relationship between negative parenting and failures to wait differed depending on child DRD4 exon III VNTR genotype.
Table 3.
Children’s DRD4 Exon III VNTR Genotype, Positive and Negative Parenting variables, and Their Interaction as Predictors of Children’s Effortful Control Failures.
| Overall Model | Change Statistics | |||||||
|---|---|---|---|---|---|---|---|---|
| Df | R2 | F | Cohen’s F | Df | ΔR2 | ΔF | B | |
| Step 1 | 3,375 | .10 | 13.76*** | .11 | ||||
| PPVT | −.002*** | |||||||
| Gender | −.070*** | |||||||
| Age | −.004 | |||||||
| Step2 | 6,372 | .14 | 9.97*** | .05 | 3,372 | .04 | 5.67** | |
| PPVT | −.002** | |||||||
| Gender | −.073*** | |||||||
| Age | −.003 | |||||||
| DRD4 Genotype | .008 | |||||||
| Positive Parenting | −.016 | |||||||
| Negative Parenting | .286** | |||||||
| Step 3 | 8,370 | .16 | 8.56*** | .02 | 2,370 | .02 | 3.87* | |
| PPVT | −.002** | |||||||
| Gender | −.068*** | |||||||
| Age | −.003 | |||||||
| DRD4 Genotype | .007 | |||||||
| Positive Parenting | −.010 | |||||||
| Negative Parenting | .111 | |||||||
| DRD4 Genotype × Positive parenting | −.012 | |||||||
| DRD4 Genotype × Negative Parenting | .408* | |||||||
p < .001,
p < .01,
p < .05
Note: PPVT, gender and age are included as covariates. PPVT = Peabody Picture Vocabulary Test, DRD4 genotype = DRD4 exon III VNTR status coded as 7-repeat absent = 0, 7-repeat present = 1, Gender coded as male = 1, female = 2.
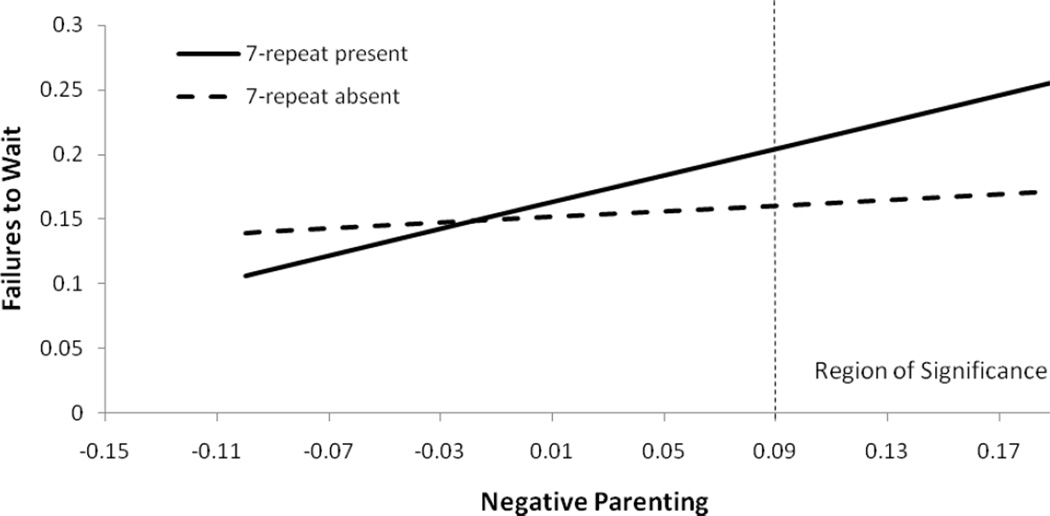
To further understand the nature of the interaction, we plotted estimated levels of failures to wait across estimated levels of negative parenting for children with and without the 7-repeat allele (adjusted for other variables in the model, see Figure 1). For children with at least one copy of the 7-repeat allele, higher levels of negative parenting were associated with more failure to wait (b = .53, SE = .16, p < .01); however, for children without a copy of the 7-repeat allele, negative parenting and failures to wait were essentially unassociated (b = .09, SE = .12, p = .43)
Figure 1.
Relationship between negative parenting aggregate and child EC failures by DRD4 exon III VNTR genotype. Note: The line on the X axis at .09, derived from the Johnson-Neyman technique (Johnson & Fay, 1950), indicates the value of negative parenting at and above which the two DRD4 genotype groups differ significantly (p < .05) in terms of EC failures.
To better determine whether the pattern of genetic moderation obtained was consistent with a diathesis-stress or differential susceptibility model, Hayes and Matthes’ guidelines (Hayes & Matthes, 2009) were used for testing regions of significance in two-way interactions in multiple linear regression according to the Johnson-Neyman technique (Johnson & Fay, 1950). This procedure uses the asymptotic variances, covariances, and other regression parameters to determine the upper and lower boundaries of the focal predictor variable at which groups representing a multi-level moderator are significantly different (p < .05) in terms of the outcome of interest. In the present case, DRD4 genotype was the focal predictor variable and the moderator was negative parenting. Thus, testing regions of significance shows which levels of negative parenting (if any) are differentially associated with EC failures for the two genotype groups. For example, the two genotype groups could differ in EC when negative parenting is low, moderate, or high, or at multiple levels of negative parenting. If differences in EC failures are evident only at high levels of negative parenting, this would favor diathesis-stress models. However, if children with a 7-repeat also show significantly lower levels of EC failures when negative parenting is especially low, this would support differential susceptibility.
The degree of negative parenting at which group differences in EC emerged is shown in Figure 1. At levels of negative parenting greater than .09, which is comparable to 1 standard deviation above the mean of our sample, children with the 7-repeat allele demonstrated significantly more failures to wait than those without a 7-repeat allele (t(370) = 1.97, p < .05). In contrast, at levels of negative parenting below .09, children with or without a 7-repeat allele did not differ in failures to wait. Thus, group differences in EC failures emerged only at relatively high levels of negative parenting.
Discussion
We examined whether parenting behaviors were associated with the development of children’s effortful control (EC) and whether the relationship between parenting behaviors and EC was moderated by children’s DRD4 exon III VNTR genotype. Consistent with the extant literature on parenting and EC (Karreman et al., 2008; Lengua et al., 2007; Valiente et al., 2006) we found bivariate associations between positive and negative parenting and children’s EC. Although positive parenting was correlated with children’s EC, this association was no longer significant in a full model including negative parenting and interaction terms reflecting parenting-DRD4 genotype effects. Additionally, negative parenting appeared most relevant in children with putative genetic risk for low EC; indeed, negative parenting was essentially unrelated to children’s EC when children did not have a 7-repeat variant of the DRD4 exon III VNTR. Our findings indicate that children’s genetic influences on EC may be critical moderators of the influence of parenting on this important trait.
The processes by which parenting modulates the influence of the DRD4 exon III VNTR on children’s EC is unclear. However, it has been argued that dopaminergic transmission plays a key role in cognitive flexibility and in reward and punishment (e.g., Ashby, Isen, & Turken, 1999; Robbins & Everitt, 1999). Such cognitive flexibility likely either promotes, or can be considered a core feature of, the development of children’s EC. While speculative, it is possible that the influence of 7-repeat of this gene on children’s cognitive flexibility, by virtue of its detrimental effects on dopaminergic neurotransmission, is more readily disrupted by negative contextual factors such as adverse parenting. For instance, while positive parenting likely corresponds to positive emotionality and dopamine release in children, negative parenting could result in less dopaminergic transmission potentially exacerbating the pre-existing dopaminergic deficit in children with the 7-repeat allele. As noted previously, relative to other DRD4 variants, the 7-repeat variant has functional effects including decreased signal transduction efficiency (Asghari et al., 1995) and translational efficiency (Schoots & Van Tol, 2003). It is possible that these biological consequences only become salient in the presence of environmental risk. However, we emphasize that our findings are in need of replication before further speculation on the processes that underlie the obtained moderation effect is justified.
Belsky and Pluess (2009), among others (Ellis et al., 2011), posit that specific genetic polymorphisms convey a general susceptibility to the influence of environmental factors, rather than risk or resilience. To support such a model in the present case, children with a 7-repeat of the DRD4 would have to have shown both relatively strong and relatively weak EC, depending upon the environmental context (i.e., parenting), compared to those without this putative marker of genetic sensitivity. However, our results were not supportive; tests of regions of significance showed that the two DRD4 genotype groups differed significantly on EC failures when negative parenting was relatively high, with those children with a 7-repeat showing significantly more EC failures than those without. When negative parenting was low, the genotype groups were not significantly different on EC. Furthermore, child DRD4 genotype was not moderated by positive parenting practices in a full model including positive and negative parenting. Our findings are therefore most consistent with a diathesis-stress model (Monroe & Simons, 1991) of the relationship between DRD4 genotype, negative parenting, and children’s EC, such that the 7-repeat appeared to convey greater vulnerability to the effects of negative parenting. However, it is important to note that we examined a single genetic variant in the present study, even though multiple genes likely influence EC. It is possible that other polymorphisms function as markers of differential susceptibility in shaping children’s EC, a possibility that should be tested in future studies of a broader array of candidate genes. It is also important to note that this gene may enhance susceptibility to both positive and negative environments with respect to predicting phenotypes other than EC (e.g., Bakermans-Kranenburg, & van Ijzendoorn, 2006).
Our results were inconsistent with previous findings from Sheese and colleagues (2007), who found that parenting and children’s DRD4 exon III VNTR genotype did not interact to predict EC. These inconsistencies likely result from methodological differences between the two studies, including the larger sample size and the use of laboratory measures of EC, rather than parent reports, in our study. Correlations between laboratory observations and parent reports of temperament are typically modest (e.g., Durbin, 2010), suggesting that the method of assessment is important. While a debate of the merits of laboratory versus parent- reports of child temperament is beyond the scope of this paper, laboratory assessments may provide a more objective index of child EC exhibited under standardized conditions.
In addition to the use of standardized observational measures of EC, the present study had several additional strengths, including a relatively large sample size for a laboratory-based genetic study, and the consideration of both contextual and genetic influences on child EC. However, our study also has several limitations; first, although EC is likely a multifaceted construct (Rothbart et al., 2001), our measure of EC was relatively narrow, consisting of only two tasks that emphasized the inhibitory control aspects of the broader construct. Future studies seeking to examine the influence of the DRD4 polymorphisms and parenting on EC would benefit from the use of a more diverse array of tasks tapping all aspects of EC (e.g., Kochanska et al., 2000). Second, research is unclear as to whether population stratification creates spurious false positive associations in ethnically homogenous populations, such as that used in the current study (Hutchison, Stallings, McGeary & Bryan, 2004). Similarly, the use of a community sample of only Caucasian participants limits the generalizability of our findings to other populations. Furthermore, as is the risk with any genetic association study, there is a possibility that the DRD4 gene exists in linkage disequilibrium with another gene which is instead responsible for the observed relationships.
Finally, the direction and mechanism of causality is unclear. Given that we report cross-sectional rather than longitudinal data, it is impossible to determine whether parenting behaviors predict EC or whether EC predicts parenting. Some research supports the notion that parenting influences children’s EC in a unidirectional manner (e.g., Eisenberg et al., 2005). However, it is likely that parenting and EC have a reciprocal relationship such that both influence one another over time (e.g., Eisenberg et al., 1999). For example, in the present case, it is plausible that children with poor EC elicit greater levels of negative parenting. Furthermore, considering that parenting is likely at least partially genetically influenced, the observed moderation may reflect a gene-gene interaction in which parenting acts as a proxy for a second gene that interacts with DRD4 to predict EC (Rutter et al., 2006). In future work, we hope to examine the relationship between parenting styles, parental genetics, child genetics, and EC longitudinally to determine how these variables unfold over time.
Despite these limitations, the current study has important implications for the development of early-emerging EC and for childhood outcomes. As previously discussed, EC has been consistently associated with social and psychological adjustment (Eisenberg et al., 2004) and has also been shown to moderate the relationship between contextual risk factors and psychopathology (e.g., Lengua, Bush, Long, Kovacs & Trancik, 2008). Our findings indicate that individuals with the 7-repeat allele of the DRD4 exon III VNTR who are exposed to high levels of negative parenting may be at particularly high risk for deficits in EC, thus increasing the likelihood that such children are on an early trajectory toward negative outcomes. While our findings should be considered tentative until replication, they indicate several potential markers of early risk for deficits in EC, some of which would be potentially amenable to preventative strategies (i.e., parenting). In particular, our findings suggest that such strategies might be particularly critical for genetically vulnerable children.
To summarize, we found that child DRD4 exon III VNTR genotype moderated the relationship between parenting behaviors and EC, indicating that some children may be especially vulnerable to negative parenting with respect to the development of EC. Despite its limitations, our study contributes to the understanding of how genetics and contextual factors interact in the development of EC in childhood.
Acknowledgments
This research was supported by a Young Investigator award from NARSAD and an Early Researcher Award from the Ontario Ministry of Research and Innovation to Elizabeth P. Hayden, a GCRC Grant no. M01-RR10710 to Stony Brook University from the National Center for Research Resources, and a National Institute of Mental Health grant R01 MH069942 to Daniel N. Klein.
Contributor Information
Heather J. Smith, University of Western Ontario
Haroon I. Sheikh, University of Western Ontario
Margaret W. Dyson, Stony Brook University
Thomas M. Olino, Stony Brook University
Rebecca S. Laptook, Stony Brook University
C. Emily Durbin, Northwestern University.
Elizabeth P. Hayden, University of Western Ontario
Shiva M. Singh, University of Western Ontario
Daniel N. Klein, Stony Brook University
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA, US: Sage Publications, Inc.; 1991. [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HHM. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken U. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–550. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Auerbach JG, Benjamin J, Faroy M, Geller V, Ebstein R. DRD4 related to infant attention and information processing: A developmental link to ADHD? Psychiatric Genetics. 2001;11:31–35. doi: 10.1097/00041444-200103000-00006. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH, Pijlman FTA, Mesman J, Juffer F. Experimental evidence for differential susceptibility: Dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddlers’ externalizing behavior in a randomized controlled trial. Developmental Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: Unexpected differences between rodents and primates. Trends in Neurosciences. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Blair C. School readiness: Integrating cognition and emotion in a neurobiological conception of children’s functioning at school entry. American Psychologist. 2002;57:111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Boy C, Klimke A, Holschbach M, Herzog H, Muhlensiepen H, Kops ER, Muller-Gartner HW. Imaging dopamine D4 receptors in the living primate brain: A positron emission tomography study using the novel D1/D4 antagonist [11C] SDZ GLC 756. Synapse. 1998;30:341–350. doi: 10.1002/(SICI)1098-2396(199812)30:4<341::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ. Individual differences in inhibitory control and children’s theory of mind. Child Development. 2001;72:1032–1053. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode model of self-regulation and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin. 2008;134:912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congdon E, Lesch KP, Canli T. Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: Implications for impulsivity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:27–32. doi: 10.1002/ajmg.b.30557. [DOI] [PubMed] [Google Scholar]
- Deth RC, Kuznetsova A, Mostafa W. Attention related signalling activities of the D4 dopamine receptor. In: Posner MI, editor. Cognitive neuroscience of attention. New York: Guilford; 2004. pp. 269–282. [Google Scholar]
- Ding YC, Chi HC, Grady DL, Morishima A, Kidd JR, Kidd KK, Moyzis RK. Evidence of positive selection acting at the human dopamine receptor D4 gene locus. Proceedings of the National Academy of Science. 2002;99:309–314. doi: 10.1073/pnas.012464099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L, Dunn D. Peabody Picture Vocabulary Test. 4th edition. Bloomington, MN: Pearsons; 2007. [Google Scholar]
- Durbin CE. Modeling temperamental risk for depression using developmentally sensitive laboratory paradigms. Child Development Perspectives. 2010;4:168–173. [Google Scholar]
- Egeland B, Weinfield N, Hiester M, Lawrence C, Pierce S, Chippendale K. Teaching tasks administration and scoring manual. University of Minnesota; 1995. [Google Scholar]
- Eisenberg N, Fabes RA, Murphey BC. Relations of shyness and low sociability to regulation and emotion. Journal of Personality and Social Psychology. 1995;68:505–517. doi: 10.1037//0022-3514.68.3.505. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Shepherd SA, Guthrie IK, Murphy BC, Reiser M. Parental reactions to children’s negative emotions: Longitudinal relations to quality of children’s social functioning. Child Development. 1999;70:513–534. doi: 10.1111/1467-8624.00037. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Gershoff ET, Fabes RA, Shepard SA, Cumberland AJ, Losoya SH, Murphy BC. Mothers’ emotional expressivity and children’s behavior problems and social competence: Mediation through children’s regulation. Developmental Psychology. 2001;37:475–490. doi: 10.1037//0012-1649.37.4.475. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Smith CL, Sadovsky A, Spinrad TL. Effortful Control. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory and application. New York: Guilford; 2004. pp. 259–282. [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Cumberland A, Liew J, Reiser M, Zhou Q, Losoya S. Longitudinal relationships of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control and externalizing problems: A three-wave longitudinal study. Child Development. 2005;76:1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Biological sensitivity to context. Current Directions in Psychological Science. 2008;17:183–187. [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Hyde JS, Goldsmith HH, Van Hulle CA. Gender differences in temperament: A meta-analysis. Psychological Bulletin. 2006;132:33–72. doi: 10.1037/0033-2909.132.1.33. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Doyle AE, Mick E, Biederman J. Meta-analysis of the association between the 7-repeat allele of the dopamine D4 receptor gene and attention deficit hyperactivity disorder. American Journal of Psychiatry. 2001;158:1052–1057. doi: 10.1176/appi.ajp.158.7.1052. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith HH, Reilly J, Lemery KS, Longley S, Prescott A. Laboratory Temperament Assessment Battery: Preschool version. 1995 Unpublished manuscript. [Google Scholar]
- Grusec JE, Goodnow JJ. Impact of parental discipline methods on the child's internalization of values: A reconceptualization of current points of view. Developmental Psychology. 1994;30:4–19. [Google Scholar]
- Guo SW, Thompson EA. Performing the exact test of Hardy-Weinburg proportion for multiple alleles. Biometrics. 1992;48:361–372. [PubMed] [Google Scholar]
- Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behavioral Research Methods. 2009;41:924–936. doi: 10.3758/BRM.41.3.924. [DOI] [PubMed] [Google Scholar]
- Hoffmann ML. Empathy and moral development: Implications for caring and justice. Cambridge, England: Cambridge University Press; 2000. [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. 1975 Unpublished manuscript. [Google Scholar]
- Hutchison KE, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130:66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Johnson PO, Fay LC. The Johnson–Neyman technique, its theory and application. Psychometrika. 1950;15:349–367. doi: 10.1007/BF02288864. [DOI] [PubMed] [Google Scholar]
- Karreman A, van Tuijl C, van Aken MAG, Dekovic M. Parenting and self-regulation in preschoolers: A meta-analysis. Infant and Child Development. 2006;15:561–579. [Google Scholar]
- Karreman A, van Tuijl C, van Aken MAG, Dekovic M. Parenting, coparenting, and effortful control in preschoolers. Journal of Family Psychology. 2008;22:30–40. doi: 10.1037/0893-3200.22.1.30. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Knaack A. EC as a personality characteristic of young children: Antecedents, correlates and consequences. Journal of Personality. 2003;71:1087–1112. doi: 10.1111/1467-6494.7106008. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Kramer UM, Rojo N, Schule R, Cunillera T, Schols L, Marco-Pallares J, Munte TF. ADHD candidate gene (DRD4 exon III) affects inhibitory control in a healthy sample. BMC Neuroscience. 2009;10:150. doi: 10.1186/1471-2202-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Doelger L, Goldsmith HH. Genetic relations between effortful and attentional control and symptoms of psychopathology in middle childhood. Infant and Child Development. 2008;17:365–385. doi: 10.1002/icd.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Bush NR, Long AC, Kovacs EA, Trancik AM. Effortful control as a moderator of the relation between contextual risk factors and growth in adjustment problems. Development and Psychopathology. 2008;20:509–528. doi: 10.1017/S0954579408000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Honorado E, Bush NR. Contextual risk and parenting as predictors of effortful control and social competence in preschool children. Journal of Applied Developmental Psychology. 2007;28:40–55. doi: 10.1016/j.appdev.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis–stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in Neurobiology. 2002;571:1–31. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: associations in a large community sample. Journal of Abnormal Psychology. 2010;119:468–478. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Fan J. Attention as an organ system. In: Pomerantz JR, editor. Topics in Intergrative Neuroscience. New York: Cambridge University Press; 2005. [Google Scholar]
- Propper C, Willoughby M, Halpern CT, Carbone MA, Cox M. Parenting quality, DRD4 and the prediction of externalizing and internalizing behaviors in early childhood. Developmental Psychobiology. 2007;49:619–632. doi: 10.1002/dev.20249. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Motivation and reward. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental neuroscience. San Diego, CA: Academic Press; 1999. pp. 1246–1260. [Google Scholar]
- Rothbart MK. Temperament, development and personality. Current Directions in Psychological Science. 2007;16:207–212. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL. Temperament and social behavior in childhood. Merrill-Palmer Quarterly. 1994;40:21–39. [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL, Fisher P. Investigations of temperament at three to seven years: The children’s behavior questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ellis LK, Rueda MR, Posner MI. Developing mechanisms of temperamental effortful control. Journal of Personality. 2003;71:1113–1143. doi: 10.1111/1467-6494.7106009. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. Attentional control and self-regulation. In: Baumeister RF, Vohs KD, editors. Handbook of self-regulation: Research, theory and application. New York: Guilford; 2004. pp. 283–300. [Google Scholar]
- Rutter ML. Nature-nurture integration: The example of antisocial behavior. American Psychologist. 1997;52:390–398. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Schachar R, Mota VL, Logan GD, Tannock R, Klim P. Confirmation of an inhibitory control deficit in attention deficit/hyperactivity disorder. Journal of Abnormal Child Psychology. 2000;28:227–235. doi: 10.1023/a:1005140103162. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DHC. Association of DRD4 with attention problems in normal childhood development. Psychiatric Genetics. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HHM. The human dopamine D4 receptor repeat sequences modulate expression. The Pharmacogenomics Journal. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Sheese BE, Voelker PM, Rothbart MK, Posner MI. Parenting quality interacts with genetic variation in dopamine receptor D4 to influence temperament in early childhood. Development and Psychopathology. 2007;19:1039–1046. doi: 10.1017/S0954579407000521. [DOI] [PubMed] [Google Scholar]
- Valiente C, Eisenberg N, Spinrad TL, Reiser M, Cumberland A, Losoya SH, Liew J. Relations among mothers’ expressivity, children’s effortful control and their problem behaviors: A four year longitudinal study. Emotion. 2006;6:459–472. doi: 10.1037/1528-3542.6.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HHM. Folding efficacy is rate-limiting in dopamine D4 receptor biogenesis. The Journal of Biological Chemistry. 2005;280:19350–19357. doi: 10.1074/jbc.M414043200. [DOI] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Bakermans-Kranenburg MJ. DRD4 7-repeat polymorphism moderates the association between maternal unresolved loss or trauma and infant disorganization. Attachment and Human Development. 2006;8:291–307. doi: 10.1080/14616730601048159. [DOI] [PubMed] [Google Scholar]
- Wang E, Ding YC, Flodman P, Kidd JR, Kidd KK, Grady DL, Moyzis RK. The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. The American Journal of Human Genetics. 2004;74:931–944. doi: 10.1086/420854. [DOI] [PMC free article] [PubMed] [Google Scholar]