Figure 6.
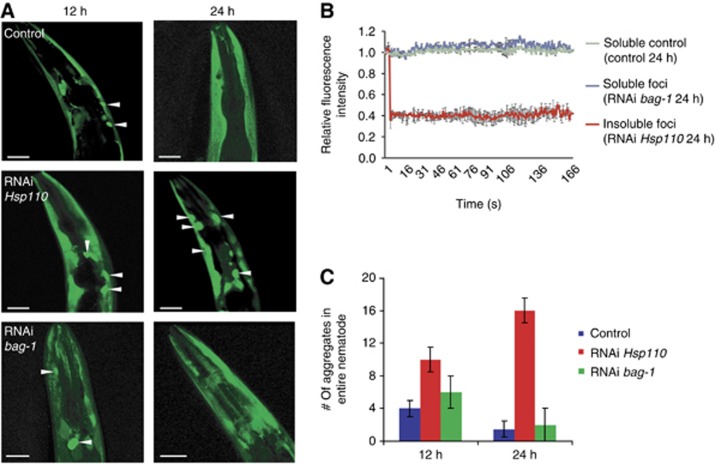
Knock-down of Hsp110 results in the persistence of heat shock-induced luciferase. (A) Luciferase-YFP expressing nematodes were heat-shocked at 35°C for 1 h and then transferred onto RNAi plates (C30C11.4 (Hsp110), bag-1 or empty vector (L4440) as control) and returned to 20°C. The aggregation propensity of luciferase-YFP was monitored during the indicated recovery time 12 h (left) and 24 h (right) post heat shock. For each condition, 20 nematodes were analysed. Representative images of the foci (white arrows) of the head region are shown. The two different time points depict different animals. The aggregation propensity however was indistinguishable between animals treated with the same RNAi and heat-shock conditions. The scale bars are 25 μM. (B) FRAP analysis demonstrates that foci of the Hsp110 knock-down are immobile aggregates (red), whereas the luciferase-YFP of the control (blue) or upon knock-down of bag-1 is soluble (green). FRAP was performed on three different animals of each genetic background. The graphs represent the average recovery of fluorescence and the variations are depicted by the error bars. (C) Quantification of luciferase-YFP foci of (A) of the whole nematode 12 and 24 h post heat shock.