Abstract
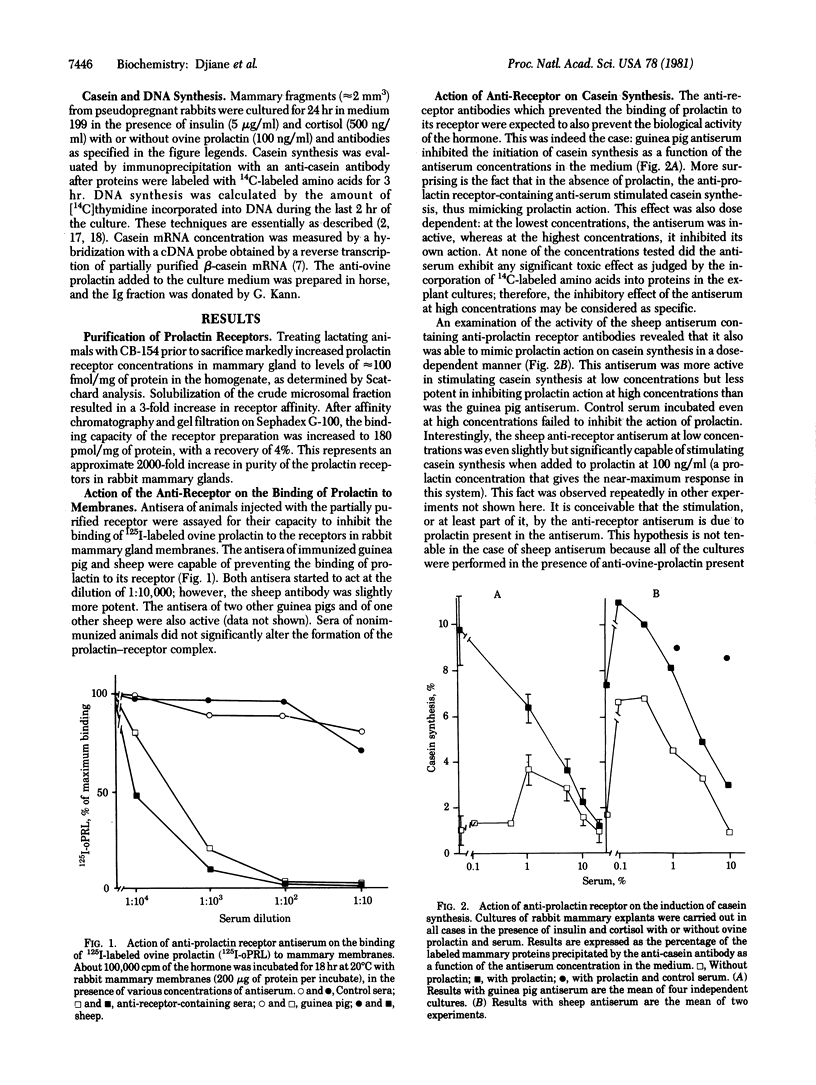
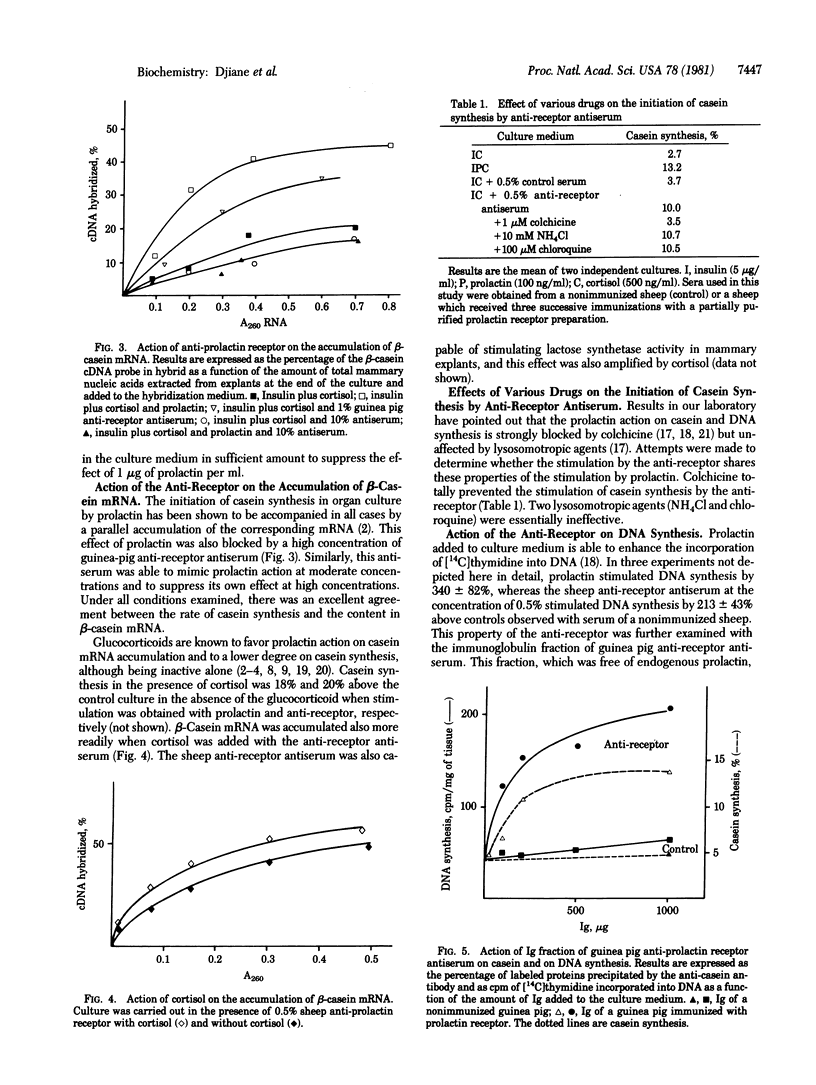
Prolactin receptors were partially purified from rabbit mammary gland membranes by using an affinity chromatography technique. Antibodies against this prolactin receptor preparation were obtained in guinea pig and sheep. Both antisera were able to inhibit the binding of 125I-labeled ovine prolactin to rabbit mammary gland membranes. When added to culture media of rabbit mammary explants, the anti-prolactin receptor antiserum inhibited the capacity of prolactin to initiate casein synthesis and casein mRNA accumulation as a function of the antiserum concentration. However, in the absence of prolactin, both antisera (guinea pig and sheep) at moderate concentrations were capable of mimicking prolactin action on casein gene expression and on DNA synthesis. At higher concentrations, the anti-prolactin receptor antibodies inhibited their own actions. Several characteristics of the prolactin effect were also observed with the anti-prolactin receptor antibody: the stimulatory effect of the antibody was amplified by glucocorticoids; colchicine, which was capable of blocking prolactin action, also prevented the induction by the antibody. Lysosomotropic agents, which do not interfere with prolactin action, did not alter the response observed with the antibody. These results indicate that an anti-prolactin receptor antibody can mimic two major actions of prolactin obtained in mammary explant culture and suggests that the prolactin molecule is not required beyond the initial binding to its receptor.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin D., Jr, Terris S., Steiner D. F. Characterization of insulin-like actions of anti-insulin receptor antibodies. Effects on insulin binding, insulin degradation, and glycogen synthesis in isolated rat hepatocytes. J Biol Chem. 1980 May 10;255(9):4028–4034. [PubMed] [Google Scholar]
- Bohnet H. G., Shiu R. P., Grinwich D., Friesen H. G. In vivo effects of antisera to prolactin receptors in female rats. Endocrinology. 1978 Jun;102(6):1657–1661. doi: 10.1210/endo-102-6-1657. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Tell G. P. Insulin-like activity of concanavalin A and wheat germ agglutinin--direct interactions with insulin receptors. Proc Natl Acad Sci U S A. 1973 Feb;70(2):485–489. doi: 10.1073/pnas.70.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech M. P., Lawrence J. C., Jr, Lynn W. S. Hexose transport in isolated brown fat cells. A model system for investigating insulin action on membrane transport. J Biol Chem. 1974 Sep 10;249(17):5421–5427. [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Djiane J., Clauser H., Kelly P. A. Rapid down-regulation of prolactin receptors in mammary gland and liver. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1371–1378. doi: 10.1016/0006-291x(79)91187-2. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P., Kelly P. A. Evolution of prolactin receptors in rabbit mammary gland during pregnancy and lactation. Endocrinology. 1977 May;100(5):1348–1356. doi: 10.1210/endo-100-5-1348. [DOI] [PubMed] [Google Scholar]
- Djiane J., Durand P. Prolactin-progesterone antagonism in self regulation of prolactin receptors in the mammary gland. Nature. 1977 Apr 14;266(5603):641–643. doi: 10.1038/266641a0. [DOI] [PubMed] [Google Scholar]
- Flier J. S., Kahn C. R., Roth J., Bar R. S. Antibodies that impair insulin receptor binding in an unusual diabetic syndrome with severe insulin resistance. Science. 1975 Oct 3;190(4209):63–65. doi: 10.1126/science.170678. [DOI] [PubMed] [Google Scholar]
- Ganguly R., Ganguly N., Mehta N. M., Banerjee M. R. Absolute requirement of glucocorticoid for expression of the casein gene in the presence of prolactin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6003–6006. doi: 10.1073/pnas.77.10.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdebine L. M., Djiane J. Effects of lysomotropic agents, and of microfilament- and microtubule-disrupting drugs on the activation of casein-gene expression by prolactin in the mammary gland. Mol Cell Endocrinol. 1980 Jan;17(1):1–15. doi: 10.1016/0303-7207(80)90099-4. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Effect of various lysosomotropic agents and microtubule disrupting drugs on the lactogenic and the mammogenic action of prolactin. Eur J Cell Biol. 1980 Oct;22(2):755–760. [PubMed] [Google Scholar]
- Houdebine L. M. Effects of prolactin and progesterone on expression of casein genes. Titration of casein mRNA by hybridization with complementary DNA. Eur J Biochem. 1976 Sep;68(1):219–225. doi: 10.1111/j.1432-1033.1976.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jarrett D. B., Roth J., Kahn C. R., Flier J. S. Direct method for detection and characterization of cell surface receptors for insulin by means of 125I-labeled autoantibodies against the insulin receptor. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4115–4119. doi: 10.1073/pnas.73.11.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson F. A., Van Obberghen E., Grunfeld C., Kahn C. R. Desensitization of the insulin receptor at an early postreceptor step by prolonged exposure to antireceptor antibody. Proc Natl Acad Sci U S A. 1979 Feb;76(2):809–813. doi: 10.1073/pnas.76.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Akanuma Y., Tsushima T., Suzuki K., Kosaka K., Kibata M. Effects of antiinsulin receptor autoantibody on the metabolism of rat adipocytes. J Clin Endocrinol Metab. 1978 Jul;47(1):66–77. doi: 10.1210/jcem-47-1-66. [DOI] [PubMed] [Google Scholar]
- Livingston J. N., Gurny P. A., Lockwood D. H. Insulin-like effects of polyamines in fat cells. Mediation by H2O2 formation. J Biol Chem. 1977 Jan 25;252(2):560–562. [PubMed] [Google Scholar]
- Matusik R. J., Rosen J. M. Prolactin induction of casein mRNA in organ culture. A model system for studying peptide hormone regulation of gene expression. J Biol Chem. 1978 Apr 10;253(7):2343–2347. [PubMed] [Google Scholar]
- Mittra I. A novel "cleaved prolactin" in the rat pituitary: Part II. In vivo mammary mitogenic activity of its N-terminal 16K moiety. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1760–1767. doi: 10.1016/s0006-291x(80)80102-1. [DOI] [PubMed] [Google Scholar]
- Mutoh H., Totsuka Y., Chou M. C., Field J. B. Effects of antibodies to bovine thyroid plasma membranes on in vitro basal and thyroid-stimulating hormone stimulation of bovine thyroid adenylate cyclase. Endocrinology. 1980 Sep;107(3):707–713. doi: 10.1210/endo-107-3-707. [DOI] [PubMed] [Google Scholar]
- Nagaiah K., Bolander F. F., Jr, Nicholas K. R., Takemoto T., Topper Y. J. Prolactin-induced accumulation of casein mRNA in mouse mammary explants: a selective role of glucocorticoid. Biochem Biophys Res Commun. 1981 Jan 30;98(2):380–387. doi: 10.1016/0006-291x(81)90851-2. [DOI] [PubMed] [Google Scholar]
- Nakhasi H. L., Quasba P. K. Quantitation of milk proteins and their mRNAs in rat mammary gland at various stages of gestation and lactation. J Biol Chem. 1979 Jul 10;254(13):6016–6025. [PubMed] [Google Scholar]
- Nolin J. M., Bogdanove E. M. Effects of estrogen on prolactin (PRL) incorporation by lutein and milk secretory cells and on pituitary PRL secretion in the postpartum rat: correlations with target cell responsiveness to PRL. Biol Reprod. 1980 Mar;22(2):393–416. doi: 10.1093/biolreprod/22.2.393. [DOI] [PubMed] [Google Scholar]
- Nolin J. M. Incorporation of endogenous prolactin by granulosa cells and dictyate oocytes in the postpartum rat: effects of estrogen. Biol Reprod. 1980 Mar;22(2):417–422. doi: 10.1093/biolreprod/22.2.417. [DOI] [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Pillion D. J., Carter-Su C. A., Pilch P. F., Czech M. P. Isolation of adipocyte plasma membrane antigens by immunoaffinity chromatography. Insulinomimetic antibodies do not bind directly to the insulin receptor or the glucose transport system. J Biol Chem. 1980 Oct 10;255(19):9168–9176. [PubMed] [Google Scholar]
- Pillion D. J., Czech M. P. Antibodies against intrinsic adipocyte plasma membrane proteins activate D-glucose transport independent of interaction with insulin binding sites. J Biol Chem. 1978 Jun 10;253(11):3761–3764. [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Blockade of prolactin action by an antiserum to its receptors. Science. 1976 Apr 16;192(4236):259–261. doi: 10.1126/science.176727. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Interaction of cell-membrane prolactin receptor with its antibody. Biochem J. 1976 Sep 1;157(3):619–626. doi: 10.1042/bj1570619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu R. P., Friesen H. G. Solubilization and purification of a prolactin receptor from the rabbit mammary gland. J Biol Chem. 1974 Dec 25;249(24):7902–7911. [PubMed] [Google Scholar]
- Terry P. M., Banerjee M. R., Lui R. M. Hormone-inducible casein messenger RNA in a serum-free organ culture of whole mammary gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2441–2445. doi: 10.1073/pnas.74.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Effects of colchicine on the transcription rate of beta-casein and 28 S-ribosomal RNA genes in the rabbit mammary gland. Biochem Biophys Res Commun. 1980 Nov 28;97(2):463–473. doi: 10.1016/0006-291x(80)90286-7. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Induction of casein synthesis by prolactin and inhibition by progesterone in the pseudopregnant rabbit treated by colchicine without any simultaneous variations of casein mRNA concentration. Eur J Biochem. 1981 Jul;117(3):563–568. doi: 10.1111/j.1432-1033.1981.tb06374.x. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of progesterone and glucocorticoids in the transcription of the beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1981 Mar;114(3):597–608. doi: 10.1111/j.1432-1033.1981.tb05186.x. [DOI] [PubMed] [Google Scholar]
- Teyssot B., Houdebine L. M. Role of prolactin in the transcription of beta-casein and 28-S ribosomal genes in the rabbit mammary gland. Eur J Biochem. 1980 Sep;110(1):263–272. doi: 10.1111/j.1432-1033.1980.tb04864.x. [DOI] [PubMed] [Google Scholar]
- Van Obberghen E., Spooner P. M., Kahn C. R., Chernick S. S., Garrison M. M., Karlsson F. A., Grunfeld C. Insulin-receptor antibodies mimic a late insulin effect. Nature. 1979 Aug 9;280(5722):500–502. doi: 10.1038/280500a0. [DOI] [PubMed] [Google Scholar]


