Abstract
The aim of the present study was to investigate changes in intramuscular triglyceride (IMTG) content and perilipin 2 expression in skeletal muscle tissue following 6 mo of endurance-type exercise training in type 2 diabetes patients. Ten obese male type 2 diabetes patients (age 62 ± 1 yr, body mass index BMI 31 ± 1 kg/m2) completed three exercise sessions/week consisting of 40 min of continuous endurance-type exercise at 75% V̇o2 peak for a period of 6 mo. Muscle biopsies collected at baseline and after 2 and 6 mo of intervention were analyzed for IMTG content and perilipin 2 expression using fiber type-specific immunofluorescence microscopy. Endurance-type exercise training reduced trunk body fat by 6 ± 2% and increased whole body oxygen uptake capacity by 13 ± 7% (P < 0.05). IMTG content increased twofold in response to the 6 mo of exercise training in both type I and type II muscle fibers (P < 0.05). A threefold increase in perilipin 2 expression was observed from baseline to 2 and 6 mo of intervention in the type I muscle fibers only (1.1 ± 0.3, 3.4 ± 0.6, and 3.6 ± 0.6% of fibers stained, respectively, P < 0.05). Exercise training induced a 1.6-fold increase in mitochondrial content after 6 mo of training in both type I and type II muscle fibers (P < 0.05). In conclusion, this is the first study to report that prolonged endurance-type exercise training increases the expression of perilipin 2 alongside increases in IMTG content in a type I muscle fiber-type specific manner in type 2 diabetes patients.
Keywords: lipid metabolism, intramuscular triglyceride, adipocyte differentiation-related protein, adipophilin, insulin sensitivity
skeletal muscle insulin resistance is a defining characteristic of type 2 diabetes and is associated with intramuscular triglyceride (IMTG) accumulation. The concept of lipid-induced insulin resistance was derived initially from cross-sectional studies that demonstrated a correlation between high IMTG concentrations and insulin resistance (30). However, trained endurance athletes are generally highly insulin sensitive despite having substantially elevated IMTG levels (13, 46). Consequently, IMTG accumulation appears to relate to insulin resistance when accompanied by a sedentary lifestyle and low oxidative capacity (5). It is postulated that a low turnover of the intramuscular lipid pool and a resultant elevation in the concentration of lipid metabolites such as diacylglycerol and ceramides mediates impairments in the insulin-signaling pathway that are responsible for reduced insulin sensitivity (28).
The benefits of prolonged endurance-type exercise training on cardiovascular and metabolic health have been well established (17) and provide a basis for prescribing exercise in the prevention and treatment of type 2 diabetes (33). Exercise training interventions that enhance oxidative capacity and improve the storage and packaging of IMTG are likely to facilitate the improvement in skeletal muscle insulin sensitivity. In accord with this, recent studies demonstrate an increase in mitochondrial density and intrinsic mitochondrial function in response to prolonged endurance-type exercise training in type 2 diabetes patients (18, 31). The impact of endurance-type exercise training on IMTG content is less clear, with studies showing an increase (10, 35), no change (6), or a decrease (4, 42) in muscle lipid storage in older obese individuals and obese type 2 diabetes patients. Changes in IMTG deposition can be assessed by the use of biochemical triglyceride (TG) extraction of muscle tissue as well as immunohistochemical analyses of oil red O-stained muscle cross-sections (43). The latter approach has shown a three- to fourfold greater lipid content in type I vs. type II muscle fibers (45). This method has been applied frequently to evaluate fiber type-specific differences in IMTG content across different populations and in response to exercise (46, 47). Therefore, it is important to utilize techniques that allow IMTG content and associated proteins to be analyzed in a muscle fiber type-specific manner.
Lipid droplets (LDs) containing IMTG are viewed as dynamic organelles that play a role in a variety of cellular functions, including lipid homeostasis and cell signaling (for recent reviews, see Refs. 11 and 12). This notion is supported by the discovery of a family of proteins associated with the phospholipid monolayer of LDs, referred to as the perilipin proteins [numbered 1 to 5 (22)]. Perilipin 1 is relatively well characterized and appears to regulate lipolysis through its interaction with lipases and coactivators at the surface of the LD (15, 48), however, its expression is reported to be limited to adipocytes and steroidogenic cells (24). Perilipin 2 [formerly known as adipocyte differentiation-related protein (ADRP) or adipophilin], on the other hand, is ubiquitously expressed and present in skeletal muscle tissue. Perilipin 2 content is closely related to IMTG concentrations and is more abundantly expressed in the type I muscle fibers (3, 27, 39). Although the exact function of perilipin 2 remains to be established, in vitro data suggest that its presence on the LD surface can limit the LD's association with adipose triglyceride lipase (ATGL) (1, 23). Therefore, TG accumulation in cells expressing perilipin 2 has been attributed to the subsequent lowering of basal lipolytic rates, which also promotes tissue insulin sensitivity (1). In agreement, human studies demonstrate that perilipin 2 gene expression is higher in insulin-sensitive vs. insulin-resistant individuals (8) and improvements in insulin-mediated glucose disposal in response to weight loss, and the pharmacological treatment of type 2 diabetes alters the expression of perilipin 2 in skeletal muscle (27, 32). However, the impact of prolonged endurance-type exercise training on fiber-type specific perilipin 2 protein expression remains to be assessed.
We hypothesized that prolonged endurance-type exercise training increases muscle lipid storage and upregulates the expression of perilipin 2. Given the importance of considering muscle fiber type when investigating IMTG and related proteins, we applied immunofluorescence microscopy techniques to investigate muscle fiber type-specific changes in IMTG, perilipin 2, and cytochrome c oxidase (COX) content following 2 and 6 mo of endurance-type exercise training in type 2 diabetes patients.
MATERIALS AND METHODS
Participants
Ten type 2 diabetes patients participated in the current study [62 ± 1 yr, body mass index (BMI) 31.2 ± 0.9 kg/m−2]. Participants had been diagnosed for ≥12 mo, were all being treated with oral blood glucose-lowering medication, and were sedentary. The study was approved by the medical ethics committee of the Virga Jesse Hospital, Belgium, and written informed consent was obtained from all participants. The patients in the current study were part of a larger project (clinical trial registration: ISRCTN32206301) investigating the impact of prolonged endurance-type exercise training in a cohort of 50 type 2 diabetes patients, as described in detail elsewhere (16).
Study Design
Participants completed a 6-mo endurance-type exercise training program. Prior to commencement of the study, and after 2 and 6 mo of the intervention, oxidative capacity, body composition, and oral glucose tolerance were assessed as described previously (16). Muscle biopsies were taken from the vastus lateralis in the morning and following an overnight fast and were analyzed for mitochondrial content, IMTG, and perilipin 2 expression. The measurements at 2 and 6 mo were performed ≥4 days after the last exercise session. Oral blood glucose and/or lipid-lowering medications were stopped 3 days prior to these measurements.
Training Intervention
Participants undertook three supervised training sessions per week in the rehabilitation center of the hospital. Each exercise session consisted of walking, cycling, and cross-country ski-type exercise and was performed for 40 min at a heart rate corresponding to exercise performed at 75% of V̇o2 peak. The relationship between V̇o2 peak and heart rate was reassessed after 2 mo, and training intensity was adjusted accordingly.
Immunohistochemistry
Muscle samples were dissected free of fat and connective tissue before being embedded in Tissue-Tek OCT Compound (Sakura Finetek Europe) and frozen in liquid nitrogen-cooled isopentane. Cryosections of 5-μm thickness were fixed in 3.7% formaldehyde and permeabilized for 5 min in 0.5% Triton X-100. Sections were then incubated for 1 h with a mouse monoclonal anti-ADRP/perilipin 2 antibody (Progen), as described previously (38, 39). As a key protein in the electron transport chain, identification of COX using a mouse monoclonal anti-OxPhos Complex IV (COX) antibody (Invitrogen) was also used as a marker of the mitochondrial network of skeletal muscle. Fiber type determination was achieved through incubation of muscle sections with mouse anti-myosin heavy chain type I (A4.840-c; developed by Dr. H. M. Blau, Developmental Studies Hybridoma Bank). Sections were then incubated with either an Alexa Fluor goat anti-mouse IgG2a 594 (for OxPhos Complex IV) or an Alex Fluor goat anti-mouse IgG1 594 (for perilipin 2) in combination with an Alexa Fluor goat anti-mouse IgM 488 (for myosin heavy chain I) (Invitrogen) for 30 min. Coverslips were mounted with a glycerol and mowiol 4-88 solution in 0.2 M Tris buffer (pH 8.5) (including 0.1% DABCO anti-fade medium). When IMTG visualization was undertaken, the neutral lipid dye oil red O staining protocol, in combination with immunofluorescence, was used (47). In this respect, oil red O was applied to sections for 30 min following incubation with antibodies for fiber type determination.
Image Capture, Processing, and Data Analysis
Image capture was performed in a blinded fashion on a wide-field Nikon E600 microscope with a 40 × 0.75 NA objective coupled to a SPOT RT KE color 3-shot charge-coupled device camera (Diagnostic Instruments) for the fiber type-specific determination of IMTG and perilipin 2. FITC (465–495 nm) and Texas Red (540–580 nm) excitation filters were used to view the Alexa Fluor 488 and 594 fluorophores, respectively. The Texas Red excitation filter was also used to view sections stained with oil red O. An inverted confocal laser scanning microscope (Leica DMIRE2; Leica Microsystems) with a 63 × 1.4 NA oil immersion objective was used to obtain digital images of mitochondria, IMTG, and perilipin 2. A Helium-Neon laser was used to excite the Alexa Fluor 594 fluorophore and oil red O, and an argon laser was used to excite the Alexa Fluor 488 fluorophore.
Image processing was undertaken using Image Pro Plus 5.1 software (Media Cybernetics). Wide-field images were used to assess fiber type-specific content of IMTG and perilipin 2. Confocal images were used to assess COX content and LD size and to visualize the subcellular distribution of perilipin 2. Fibers positively stained for myosin heavy chain I were considered type I muscle fibers, and nonstained fibers were considered type II muscle fibers. Identification of COX, IMTG, and perilipin 2 was achieved through the selection of an intensity threshold that was used uniformly for all images in that series. COX, IMTG, and perilipin 2 content was expressed as the percentage fiber area positively stained. IMTG and perilipin 2 density were expressed as number of positively stained “spots” corrected for fiber area (μm2). Mean LD size was calculated by dividing the total number of objects by the total area stained. A total of 100 ± 12, 72 ± 6, and 86 ± 7 fibers per muscle cross-section were analyzed for COX, IMTG, and perilipin 2 analysis, respectively.
Statistics
All data are expressed as means ± SE. Significance was set at the 0.05 level of confidence. Changes in whole body characteristics, exercise capacity, body composition, and insulin sensitivity were analyzed using a one-way repeated measures ANOVA, with the within-subject factor as “time” (0 vs. 2 vs. 6 mo). Changes in COX, IMTG, and perilipin 2 were assessed using a two-way repeated-measures ANOVA with two within-subject factors: “fiber” (type I vs. type II fibers) and “time” (0 vs. 2 vs. 6 mo). Significant main effects or interactions were assessed using Bonferroni adjustment post hoc analysis.
RESULTS
Participants
Participant characteristics are displayed in Table 1. Significant reductions in body mass and BMI were observed with training (P < 0.05; Table 1) and were accompanied by a reduction in relative trunk fat and leg fat percentage of 6 ± 2 and 5 ± 2% posttraining, respectively (P < 0.05). Maximal oxygen uptake demonstrated a significant increase over time (P < 0.05) and was 16 ± 3% higher after 2 mo of training (from 23.4 ± 1.5 to 27.1 ± 1.7 ml·kg−1·min−1, P < 0.01) and remained 13 ± 7% higher compared with baseline values after 6 mo of intervention (26.5 ± 1.9 ml·kg−1·min−1, P = 0.08). Although the total cohort showed significant improvements in glycemic control, as shown by reduced levels of Hb A1c (16), in this small subcohort of 10 subjects the decrease in Hb A1c following training failed to reach significance (7.0 ± 0.4, 6.7 ± 0.3, and 6.5 ± 0.2% for 0, 2, and 6 mo, respectively, P = 0.20). No significant changes in fasting plasma glucose and insulin concentrations, 2-h post-oral glucose tolerance test glucose concentrations, or homeostasis model assessment index of insulin sensitivity were observed in response to training (P > 0.05).
Table 1.
Subject characteristics
| Time |
||||
|---|---|---|---|---|
| Characteristic | Baseline | 2 Mo | 6 Mo | Effect of Time (P Value) |
| Age, yr | 62 ± 1 | |||
| Height, m | 1.72 ± 0.02 | |||
| Body weight, kg | 92.9 ± 3.4 | 91.7 ± 3.4 | 91.3 ± 3.4 | <0.05 |
| Body mass index, kg/m | 31.2 ± 0.9 | 30.8 ± 0.9 | 30.7 ± 0.9 | <0.05 |
| Insulin sensitivity | ||||
| Fasting glucose, mmol/l | 9.2 ± 0.7 | 9.0 ± 0.7 | 8.7 ± 0.7 | 0.632 |
| 2-h Glucose, mmol/l | 17.9 ± 1.6 | 16.5 ± 1.6 | 15.5 ± 1.7 | 0.367 |
| Fasting insulin, μIU/ml | 17.8 ± 2.3 | 16.8 ± 2.3 | 17.7 ± 2.1 | 0.855 |
| HOMA index | 7.5 ± 1.4 | 7.0 ± 1.5 | 7.1 ± 1.2 | 0.865 |
| Hb A1c, % | 7.0 ± 0.4 | 6.7 ± 0.3 | 6.5 ± 0.2 | 0.200 |
| Exercise capacity | ||||
| V̇o2peak, l/min | 2.15 ± 0.14 | 2.46 ± 0.15* | 2.41 ± 0.19 | <0.05 |
| V̇o2peak, ml·kg−1·min−1 | 23.4 ± 1.5 | 27.1 ± 1.7* | 26.5 ± 1.9 | <0.05 |
| Wmax (W) | 180 ± 12 | 189 ± 11 | 189 ± 11 | 0.134 |
| Body composition | ||||
| %Trunk fat | 38.4 ± 1.4 | 37.3 ± 1.7 | 35.8 ± 1.3* | <0.01 |
| %Leg fat | 23.2 ± 1.6 | 22.4 ± 1.5 | 21.8 ± 1.3 | <0.05 |
Data provided represent means ± SE (n = 10). HOMA, homeostasis model assessment; V̇o2peak, peak oxygen uptake; Wmax, maximal workload.
P < 0.05 vs. baseline.
Immunohistochemical Analysis
COX.
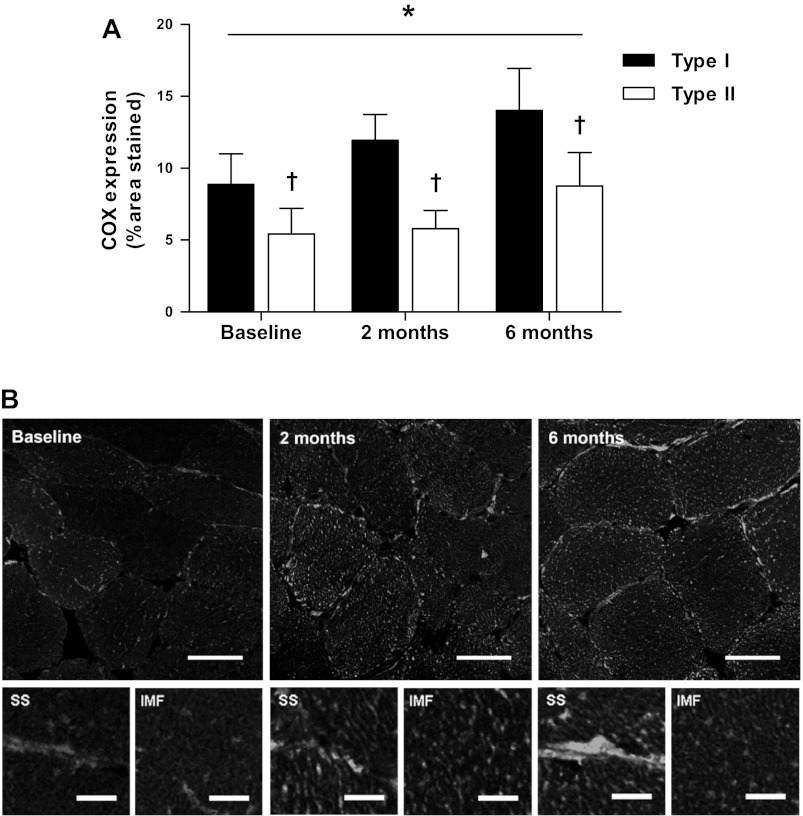
Muscle fiber type-specific COX expression was significantly greater in type I compared with type II muscle fibers at all time points (P < 0.01; Fig. 1). Six months of endurance-type exercise training induced a time-dependent increase in COX expression in both type I and type II fibers (P < 0.01). Following 6 mo of exercise training, COX expression was higher than baseline in both type I (8.9 ± 2.1 vs. 14.0 ± 2.9% fiber stained) and type II muscle fibers (5.4 ± 1.8 vs. 8.8 ± 2.4% fiber stained). The confocal micrographs of COX-stained muscle fibers at baseline, 2 mo, and 6 mo are presented in Fig. 1B. These images demonstrate greater COX density in both intermyofibrillar and subsarcolemmal regions of the muscle fibers after 6 mo of training and are most prominent in the subsarcolemmal regions.
Fig. 1.
Fiber-type specific cytochrome c oxidase (COX) expression. A: mean fiber type-specific COX expression (expressed as %fiber area stained) at baseline and following 2 and 6 mo of exercise training. Data represent means ± SE. Time effect, P = 0.006; fiber effect, P < 0.001; interaction of time and fiber, P = 0.112. *Significant effect of time; †significant difference between fiber types (P < 0.05). B, top: representative images of mitochondrial network, stained with anti-COX and viewed and quantified with confocal immunofluorescence microscopy. Scale bar, 40 μm. B, bottom: representative images of subsarcolemmal (SS) and intermyofibrillar (IMF) mitochondria areas. Scale bar, 1 μm.
IMTG.
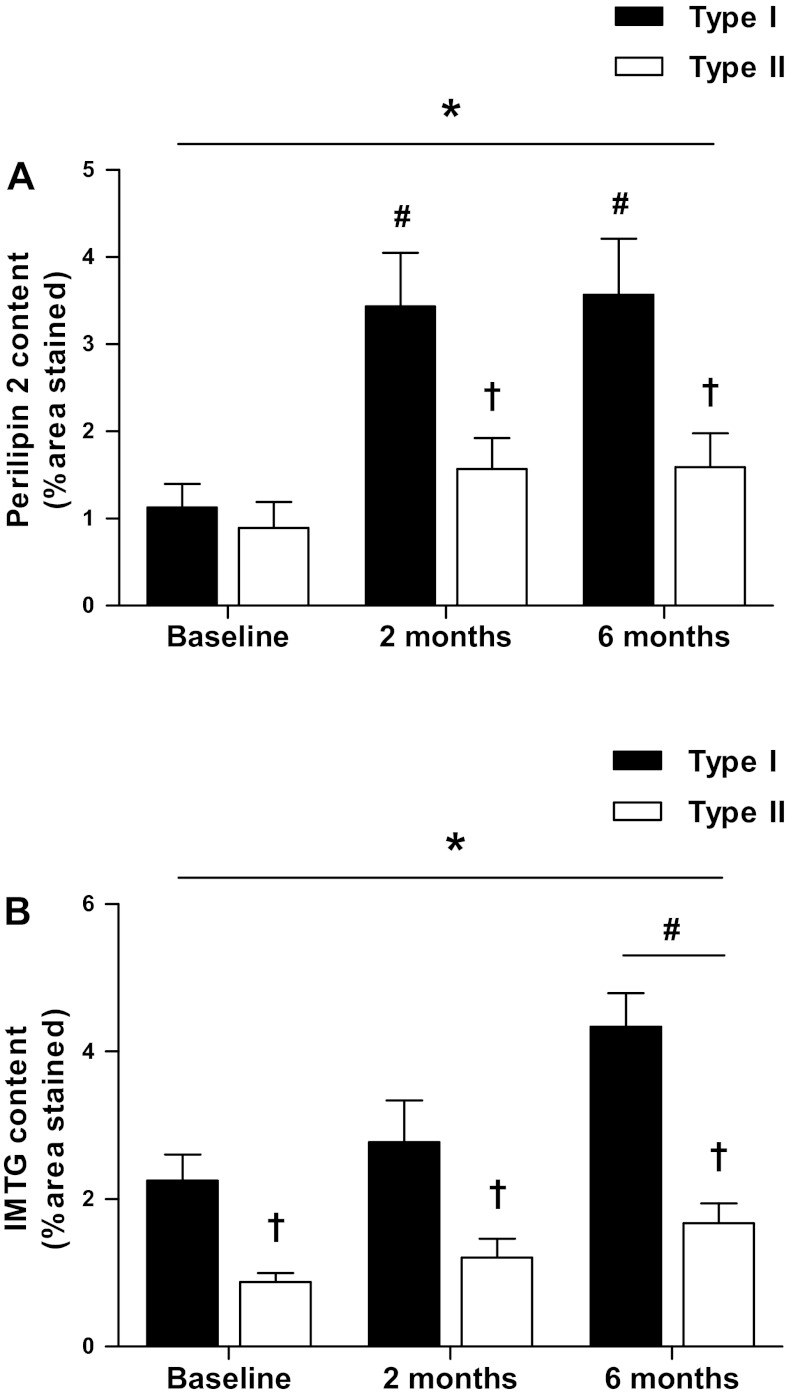
IMTG content, expressed as the area fraction stained, differed between type I and type II muscle fibers at each time point (P < 0.01; Fig. 2A). Six months of endurance-type exercise training induced a time-dependent increase in IMTG content in both type I and II muscle fibers. IMTG content had increased ∼1.9-fold in type I (2.3 ± 0.4 vs. 4.3 ± 0.5%, P < 0.01) and type II fibers (0.9 ± 0.1 vs. 1.7 ± 0.3%, P < 0.01) following 6 mo of training. The increase in IMTG content was mirrored by significant increases in IMTG density after 6 mo of training in type I fibers (0.051 ± 0.007 and 0.079 ± 0.008 LD/μm2 for 0 and 6 mo, respectively, P < 0.01) but not in type II muscle fibers (0.022 ± 0.002 and 0.031 ± 0.003 LD/μm2 for 0 and 6 mo, respectively, P = 0.056). No significant changes in IMTG content or density were apparent after 2 mo of training in either fiber type (P > 0.05). There were no differences in LD size, as determined by confocal microscopy, between fiber types or in response to endurance-type exercise training (P > 0.05).
Fig. 2.
Fiber type-specific intramuscular triglyceride (IMTG) content and perilipin 2 expression. Mean fiber type-specific perilipin 2 expression (A) and IMTG content (B) at baseline and following 2 and 6 mo of exercise training (expressed as %fiber area stained). Data represent means ± SE. A: time effect, P = 0.001; fiber effect, P < 0.001; interaction of time and fiber, P = 0.003. B: time effect, P = 0.017; fiber effect, P < 0.001; interaction of time and fiber, P = 0.01. *Significant effect of time; †significant difference between fiber types; #significant difference from baseline (P < 0.05).
Perilipin 2.
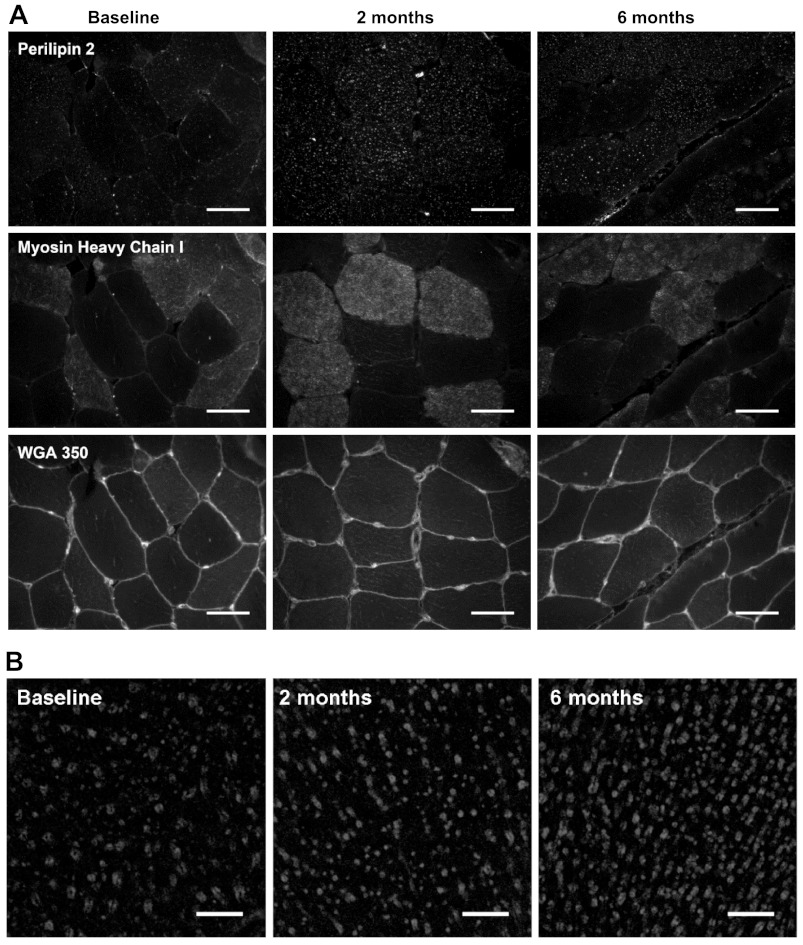
At baseline, perilipin 2 expression, calculated as the percentage of area stained, did not differ between type I and type II muscle fibers (P > 0.05; Fig. 2B). Six months of exercise training induced a time-dependent increase in perilipin 2 expression in type I muscle fibers only (P < 0.05). Compared with baseline, perilipin 2 expression in type I muscle fibers had increased approximately threefold after 2 mo of training (1.1 ± 0.3 vs. 3.6 ± 0.6%, P < 0.05), with no further increase observed after 6 mo of training. This fiber type-specific training response resulted in greater perilipin 2 expression in type I compared with type II muscle fibers after 2 and 6 mo of training (P < 0.05). Representative immunofluorescence images of perilipin 2 expression in type I and type II muscle fibers at baseline and after 2 and 6 mo of training are shown in Fig. 3. Compared with baseline, perilipin 2 density in type I fibers also increased approximately twofold after 2 and 6 mo (0.018 ± 0.003, 0.033 ± 0.004, and 0.035 ± 0.005 perilipin 2 objects/μm2 for 0, 2, and 6 mo respectively, P < 0.05), whereas perilipin 2 density in type II muscle fibers did not change (P > 0.05). Therefore, perilipin 2 density was higher in type I than type II muscle fibers after 2 and 6 mo of training only. Higher-magnification images of perilipin 2 were obtained using confocal laser scanning microscopy and are shown in Fig. 3. These images show a clear increase in perilipin 2 expression after 6 mo of training. These images demonstrate that distinct rings of perilipin 2 can be observed frequently and are more abundant after prolonged exercise training.
Fig. 3.
Immunofluorescence images of fiber type-specific perilipin 2 protein expression. A: representative images of perilipin 2 at baseline and 6 mo, stained using anti-perilipin 2 in combination with anti-myosin heavy chain type I and wheat germ agglutinin 350 (WGA350) and viewed and quantified with wide-field immunofluorescence microscopy. Scale bar, 50 μm. B: representative confocal images of perilipin 2 at baseline and 6 mo; rings of perilipin staining are clearly visible in both images. Scale bar, 10 μm.
DISCUSSION
Prolonged endurance-type exercise training is known to improve insulin-stimulated glucose uptake and glycemic control in type 2 diabetes patients (17). In this study, we demonstrate that endurance-type exercise training also increases both IMTG deposition and COX expression, which are higher in type I muscle fibers. In accord with this, we show for the first time that training induces a greater expression of perilipin 2 in type I muscle fibers.
Insulin sensitivity is enhanced by regular physical activity, which explains why significant improvements in glycemic control were observed in the previous study after 6 mo of endurance-type exercise training in a large cohort of type 2 diabetes patients (16). In the subset of participants used in this study there was no significant change in glycemic control, as was evident from the Hb A1c levels after 6 mo of training (Table 1). Nevertheless, a decline from 7.0 ± 0.4 down to 6.5 ± 0.2% in Hb A1c is of great clinical significance because it would translate into a >10% reduction in the risk of premature death, a 5–10% reduction in the risk of myocardial infarction, and an ∼20% reduction in the risk of microvascular disease (25).
Skeletal muscle oxidative capacity and whole body fatty acid oxidation are good predictors of muscle insulin sensitivity (5, 13, 14, 19). Obese individuals with insulin resistance and type 2 diabetes commonly display a reduced capacity for oxidative metabolism (2, 20, 21, 36). Thus, it is likely that increased oxidative capacity following exercise interventions is mechanistically linked to improvements in metabolic health in this population. Accordingly, we observed an ∼1.6-fold increase in COX expression in skeletal muscle following 6 mo of endurance-type exercise training (Fig. 1). The increase in COX expression in this subset of patients is in agreement with the 50% increase in COX and citrate synthase activities observed in the full cohort of patients reported previously (16). We extend on these previous data by the application of immunofluorescence microscopy, allowing us to assess oxidative capacity in a muscle fiber type-specific manner. Furthermore, we also assessed subcellular localization of the observed increases in oxidative capacity (Fig. 1). The present work shows that increases in the content of the mitochondrial enzyme COX can be observed in both the subsarcolemmal and intermyofibrillar region of the type I muscle fibers. In agreement with previous data investigating mitochondrial content following a 10-wk training intervention in type 2 diabetes patients using transmission electron microscopy (29), we show that increased COX expression is prominent in subsarcolemmal regions of type I fibers after prolonged endurance-type exercise training.
The exercise training-induced increase in skeletal muscle oxidative capacity was accompanied by an approximately twofold elevation in skeletal muscle lipid deposition in both type I and type II muscle fibers (Fig. 2). This is the first study to show a type I muscle fiber-specific increase in IMTG content following prolonged exercise training in type 2 diabetes patients. These findings tend to be in line with several recent studies demonstrating IMTG accretion coupled to increased oxidative capacity in older, obese, insulin-resistant individuals following 12–16 wk of exercise training (10, 35). Although IMTG content is already elevated in obese type 2 diabetes patients, these levels still remain below those observed in endurance-trained athletes who are highly insulin sensitive (13, 46). The high IMTG content in combination with a reduced oxidative capacity in type 2 diabetes patients likely mediates the reduction in muscle insulin sensitivity rather than merely elevated IMTG stores. Accordingly, exercise training-induced increases in mitochondrial content coupled with IMTG accretion appear to enhance insulin sensitivity. For example, a recent study has demonstrated that training-induced increases in IMTG concentrations and improvements in insulin sensitivity are coupled with a reduction in the concentration of diacylglycerol and ceramide (9). Therefore, it has been hypothesized that the process of IMTG synthesis consumes the lipid metabolites that are precursors to IMTG and impair skeletal muscle insulin signaling. In further support, the high IMTG synthesis rates observed in the period after endurance-type exercise protect against the development of insulin resistance during (intra)lipid infusion (37). The present study adds to this growing body of evidence by demonstrating greater IMTG storage and improved glycemic control in response to 6 mo of training in type 2 diabetes patients. Some studies employing a shorter training duration have failed to observe a significant increase in type 1 muscle fiber IMTG content following training in type 2 diabetic patients (26). Therefore, it is possible that a more prolonged intervention, such as the 6-mo endurance training program applied in the current study, is required before increases in IMTG deposition are observed in type 2 diabetes patients. The duration of the training intervention, in addition to the method of IMTG analysis, may also explain the discrepancy across the many studies investigating changes in IMTG content.
The increase in total IMTG content following training in the present study was accompanied by an increase in the number of LDs in type I fibers, whereas there was no change detected in LD size. This is in agreement with a previous electron microscopy study in young males and females where the increase in total IMTG content with training was due to an increase in LD density, whereas LD size remained unchanged (44). IMTG expansion through an increase in the number of smaller LDs would befit a metabolic advantage because the surface area available for the interaction of lipolytic enzymes with the regulatory proteins contained on the LD surface would be enhanced. This would maximize the capacity for rapid LD turnover, allowing more efficient lipid mobilization and therefore oxidation during exercise.
One of the regulatory proteins that reside on the surface of the LD monolayer is perilipin 2. In the current study, despite an approximatelty twofold higher IMTG concentration in the type I muscle fibers being observed (Fig. 2A), there was no difference in perilipin 2 expression between type I and type II muscle fibers prior to endurance-type exercise training (Fig. 2B). However, training induced a significant increase in perilipin 2 expression in type I muscle fibers. The perilipins are important in the packaging of LDs, and in vitro studies demonstrate that perilipin 2 expression increases cellular TG and improves insulin sensitivity. The presence of perilipin 2 at the LD surface appears to limit the association of ATGL with the LD surface, reduce basal lipolytic rates, and therefore promote TG storage (1, 23).
We show that when type 2 diabetes patients are physically active, type I muscle fibers exhibit a greater expression of perilipin 2 than type II muscle fibers. This is in agreement with our previous observations of a greater perilipin 2 expression in the type I muscle fibers of sedentary individuals and trained cyclists (39, 40). The increase in perilipin 2 expression in type I muscle fibers is likely to result in enhanced coverage of the LD surface with perilipin 2. This adaptation would limit rates of basal lipolysis and promote IMTG storage in the basal state. Furthermore, hormone-sensitive lipase translocates to perilipin 2-coated LDs during muscle contraction and adrenaline stimulation (34), and perilipin 2-associated LDs are depleted during exercise (40). We hypothesize that an increase in perilipin 2 surface coverage of the LD, along with the greater total LD surface area available and the enhanced mitochondrial density, would aid the mobilization and oxidation of the IMTG pool during exercise. This proposed improvement in the regulation of IMTG turnover both at rest and during exercise may go some way toward explaining why insulin sensitivity can be enhanced despite further accumulation of IMTG with training. However, it should be noted that neither intramuscular lipolysis nor lipid oxidation rates were assessed in the present study; therefore, further research is required to fully explore the relationship between changes in perilipin 2 expression and intramuscular lipid oxidation.
A nonexercise control group was not included in the present study; however, reductions in fat mass and improvements in V̇o2 max and muscle oxidative capacity are not seen in a similar time frame in nonexercising controls (7, 41). Therefore, we can be confident that the related changes in perilipin 2 expression and IMTG storage in the present study are specific adaptations to the exercise intervention. Because perilipin 2 is one of four perilipin proteins present in skeletal muscle, additional investigations examining other perilipins are required to fully understand the role of IMTG metabolism in the development of insulin resistance and the insulin-sensitizing effect of endurance-type training.
In conclusion, prolonged endurance-type exercise training increases intramuscular lipid storage in a muscle fiber type-dependent manner in type 2 diabetes patients. Importantly, the increase in IMTG content is accompanied by a type I muscle fiber-specific increase in perilipin 2 expression. The greater perilipin 2 expression following prolonged endurance-type exercise training in combination with increased oxidative capacity may explain the improved turnover of the skeletal muscle lipid pool with regular physical activity and likely contributes to the improvements in skeletal muscle insulin action and subsequent glycemic control.
DISCLOSURES
No conflicts of interests, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.S.S., S.O.S., A.J.W., D.H., P.D., and L.J.v.L. did the conception and design of the research; C.S.S., S.O.S., D.H., P.D., and L.J.v.L. performed the experiments; C.S.S. and S.O.S. analyzed the data; C.S.S., S.O.S., A.J.W., D.H., P.D., and L.J.v.L. interpreted the results of the experiments; C.S.S. and S.O.S. prepared the figures; C.S.S. and S.O.S. drafted the manuscript; C.S.S., S.O.S., A.J.W., D.H., P.D., and L.J.v.L. approved the final version of the manuscript; A.J.W., D.H., P.D., and L.J.v.L. edited and revised the manuscript.
ACKNOWLEDGMENTS
The antibody against myosin (human slow fibers, A4.840) used in this study was developed by Dr. H. M. Blau and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA.
REFERENCES
- 1. Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 57: 2037–2045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boushel R, Gnaiger E, Schjerling P, Skovbro M, Kraunsøe R, Dela F. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia 50: 790–796, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brasaemle D, Barber T, Wolins N, Serrero G, Blanchette-Mackie E, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997 [PubMed] [Google Scholar]
- 4. Bruce C, Kriketos A, Cooney G, Hawley J. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia 47: 23–30, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bruce CR, Anderson MJ, Carey AL, Newman DG, Bonen A, Kriketos AD, Cooney GJ, Hawley JA. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab 88: 5444–5451, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Bruce CR, Thrush AB, Mertz VA, Bezaire V, Chabowski A, Heigenhauser GJ, Dyck DJ. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab 291: E99–E107, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, Mikus CR, Myers V, Nauta M, Rodarte RQ, Sparks L, Thompson A, Earnest CP. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA 304: 2253–2262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coen PM, Dubé JJ, Amati F, Stefanovic-Racic M, Ferrell RE, Toledo FG, Goodpaster BH. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59: 80–88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dubé JJ, Amati F, Toledo FG, Stefanovic-Racic M, Rossi A, Coen P, Goodpaster BH. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54: 1147–1156, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dubé JJ, Amati F, Stefanovic-Racic M, Toledo FG, Sauers SE, Goodpaster BH. Exercise-induced alterations in intramyocellular lipids and insulin resistance: the athlete's paradox revisited. Am J Physiol Endocrinol Metab 294: E882–E888, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell 139: 855–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goodman JM. The gregarious lipid droplet. J Biol Chem 283: 28005–28009, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 52: 2191–2197, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284: 34538, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hansen D, Dendale P, Jonkers RA, Beelen M, Manders RJ, Corluy L, Mullens A, Berger J, Meeusen R, van Loon LJ. Continuous low- to moderate-intensity exercise training is as effective as moderate- to high-intensity exercise training at lowering blood HbA(1c) in obese type 2 diabetes patients. Diabetologia 52: 1789–1797, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hansen D, Dendale P, van Loon LJ, Meeusen R. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med 40: 921–940, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Hey-Mogensen M, Højlund K, Vind B, Wang L, Dela F, Beck-Nielsen H, Fernström M, Sahlin K. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia 53: 1976–1985, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol Endocrinol Metab 277: E1130–E1141, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Kelley DE, Simoneau JA. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51: 468–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48: 2751–2761, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Londos C, Brasaemle DL, Schultz CJ, Segrest JP, Kimmel AR. Perilipins, ADRP, and other proteins that associate with intracellular neutral lipid droplets in animal cells. Semin Cell Dev Biol 10: 51–58, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Manley S. Haemoglobin A1c—a marker for complications of type 2 diabetes: the experience from the UK Prospective Diabetes Study (UKPDS). Clin Chem Lab Med 41: 1182–1190, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Meex RC, Schrauwen-Hinderling VB, Moonen-Kornips E, Schaart G, Mensink M, Phielix E, Van De Weijer T, Sels JP, Schrauwen P, Hesselink MK. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes 59: 572–579, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minnaard R, Schrauwen P, Schaart G, Jorgensen JA, Lenaers E, Mensink M, Hesselink MK. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab 94: 4077–4085, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Moro C, Bajpeyi S, Smith SR. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294: E203–E213, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Nielsen J, Mogensen M, Vind BF, Sahlin K, Højlund K, Schrøder HD, Ørtenblad N. Increased subsarcolemmal lipids in type 2 diabetes: effect of training on localization of lipids, mitochondria, and glycogen in sedentary human skeletal muscle. Am J Physiol Endocrinol Metab 298: E706–E713, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia 53: 1714–1721, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phillips SA, Choe CC, Ciaraldi TP, Greenberg AS, Kong AP, Baxi SC, Christiansen L, Mudaliar SR, Henry RR. Adipocyte differentiation-related protein in human skeletal muscle: relationship to insulin sensitivity. Obes Res 13: 1321–1329, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Praet SF, van Loon LJ. Optimizing the therapeutic benefits of exercise in Type 2 diabetes. J Appl Physiol 103: 1113–1120, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Prats C, Donsmark M, Qvortrup K, Londos C, Sztalryd C, Holm C, Galbo H, Ploug T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J Lipid Res 47: 2392–2399, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Pruchnic R, Katsiaras A, He J, Kelley DE, Winters C, Goodpaster BH. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab 287: E857–E862, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54: 8–14, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest 117: 1690–1698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaw CS, Jones DA, Wagenmakers AJM. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol 129: 65–72, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Shaw CS, Sherlock M, Stewart PM, Wagenmakers AJM. Adipophilin distribution and colocalisation with lipid droplets in skeletal muscle. Histochem Cell Biol 131: 575–581, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, Wagenmakers AJ, Shaw CS. Preferential utilization of perilipin 2-associated intramuscular triglycerides during 1 h of moderate-intensity endurance-type exercise. Exp Physiol 97: 970–980, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Short KR, Vittone JL, Bigelow ML, Proctor DN, Rizza RA, Coenen-Schimke JM, Nair KS. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 52: 1888–1896, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Solomon TP, Sistrun SN, Krishnan RK, Del Aguila LF, Marchetti CM, O'Carroll SM, O'Leary VB, Kirwan JP. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 104: 1313–1319, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stellingwerff T, Boon H, Jonkers RA, Senden JM, Spriet LL, Koopman R, van Loon LJ. Significant intramyocellular lipid use during prolonged cycling in endurance-trained males as assessed by three different methodologies. Am J Physiol Endocrinol Metab 292: E1715–E1723, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tarnopolsky MA, Rennie CD, Robertshaw HA, Fedak-Tarnopolsky SN, Devries MC, Hamadeh MJ. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol 292: R1271–R1278, 2007 [DOI] [PubMed] [Google Scholar]
- 45. van Loon LJ. Use of intramuscular triacylglycerol as a substrate source during exercise in humans. J Appl Physiol 97: 1170–1187, 2004 [DOI] [PubMed] [Google Scholar]
- 46. van Loon LJ, Koopman R, Manders R, van der Weegen W, van Kranenburg GP, Keizer HA. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. Am J Physiol Endocrinol Metab 287: E558–E565, 2004 [DOI] [PubMed] [Google Scholar]
- 47. van Loon LJ, Koopman R, Stegen JH, Wagenmakers AJ, Keizer HA, Saris WH. Intramyocellular lipids form an important substrate source during moderate intensity exercise in endurance-trained males in a fasted state. J Physiol 553: 611–625, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem 284: 32116–32125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]