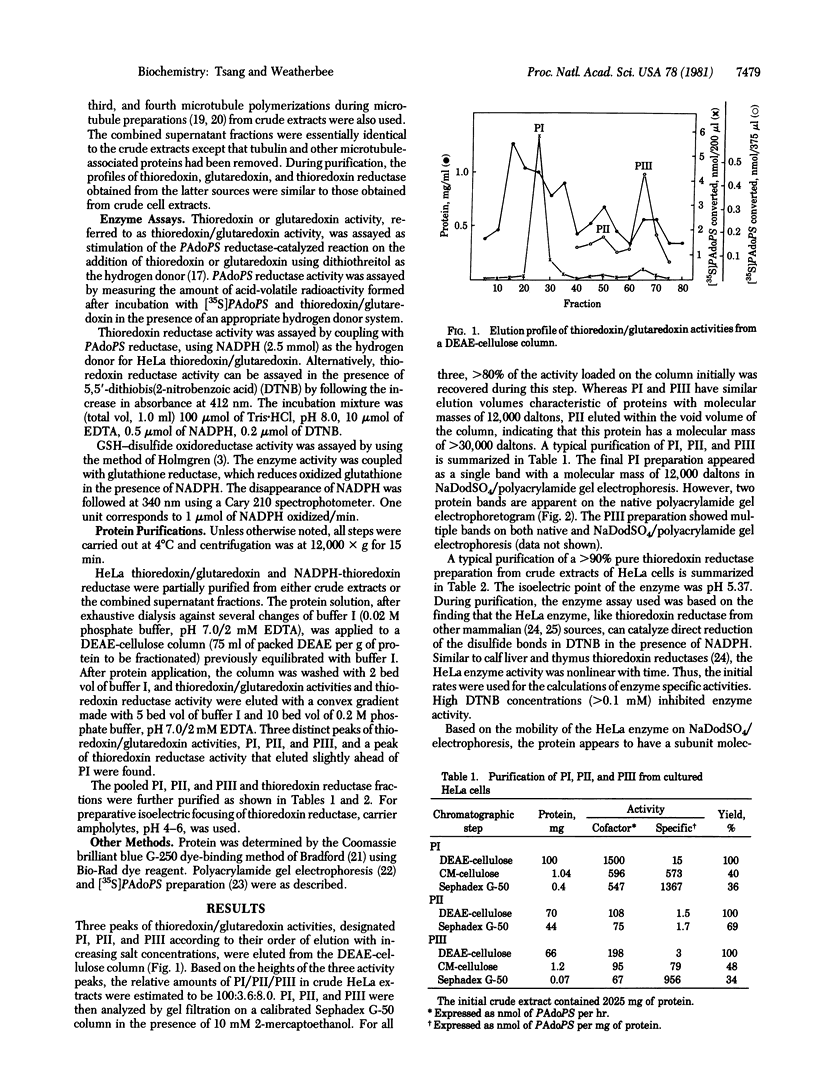
Abstract
Thioredoxin and glutaredoxin may be important in regulating cell metabolism by mediating interchanges between sulfhydryl and disulfide groups. Components of the thioredoxin/glutaredoxin system from cultured HeLa cells have been partially purified and characterized by using Escherichia coli adenosine 3'-phosphate 5'-phosphosulfate reductase, a thioredoxin/glutaredoxin-dependent enzyme on the pathway of sulfate reduction, as an assay system. In HeLa cells, a NADPH-thioredoxin reductase and three heat-labile proteins (designated PI, PII, and PIII) that have thioredoxin- or glutaredoxin-like properties are found. Both PI and PIII have molecular masses of approximately 12,000 daltons and are readily reduced by their homologous HeLa thioredoxin reductase. However, only PI can be reduced efficiently by the glutathione system and neither PI nor PIII has inherent glutathione-disulfide oxidoreductase activity. PII has a molecular mass of greater than 30,000 daltons and appears to be associated with a reductase activity. The HeLa NADPH-thioredoxin reductase has been purified to near homogeneity and found to be a 116,000-dalton flavoprotein composed of two 58,000-dalton subunits. The HeLa enzyme has low species and substrate specificity and can reduce HeLa PI and PIII, E. coli thioredoxin and glutaredoxin, and the disulfide bond in 5,5'-dithiobis(2-nitrobenzoic acid). The exact in vivo roles of the HeLa thioredoxin/glutaredoxin system remain to be determined.
Full text
PDF
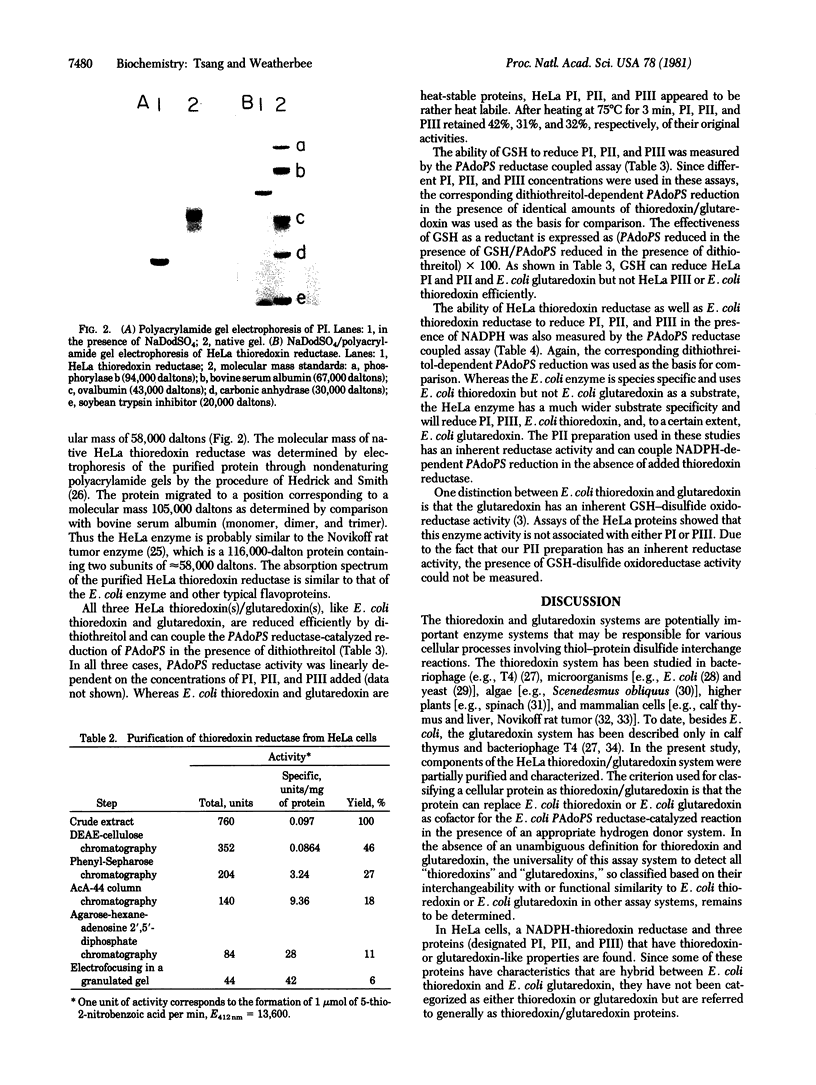

Images in this article
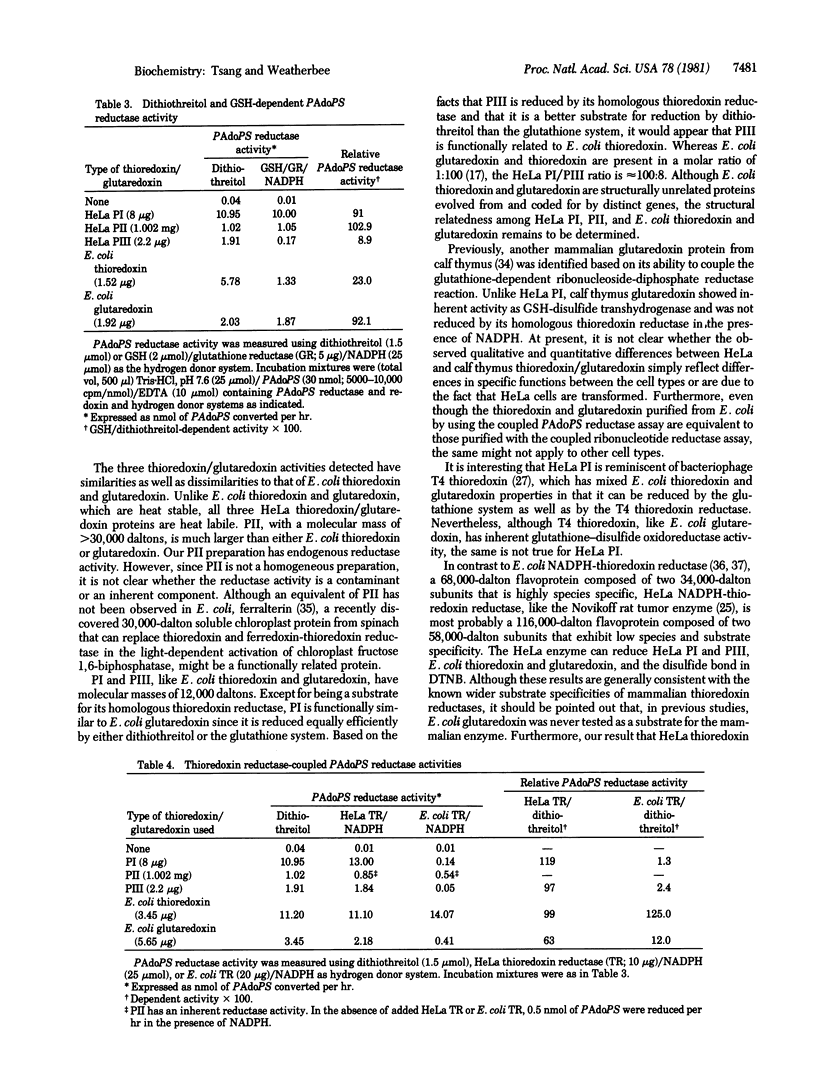
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blombäck B., Blombäck M., Finkbeiner W., Holmgren A., Kowalska-Loth B., Olovson G. Enzymatic reduction of disulfide bonds in fibrin-ogen by the thioredoxin system. I. Identification of reduced bonds and studies on reoxidation process. Thromb Res. 1974 Jan;4(1):55–75. doi: 10.1016/0049-3848(74)90203-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen C. C., McCall B. L., Moore E. C. Purification of thioredoxin reductase from the Novikoff rat tumor. Prep Biochem. 1977;7(2):165–177. doi: 10.1080/00327487708061633. [DOI] [PubMed] [Google Scholar]
- Engström N. E., Holmgren A., Larsson A., Söderhäll S. Isolation and characterization of calf liver thioredoxin. J Biol Chem. 1974 Jan 10;249(1):205–210. [PubMed] [Google Scholar]
- Freedman R. B. How many distinct enzymes are responsible for the several cellular processes involving thiol:protein-disulphide interchange? FEBS Lett. 1979 Jan 15;97(2):201–210. doi: 10.1016/0014-5793(79)80085-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. Purification of a thioredoxin system from yeast. J Biol Chem. 1970 May 10;245(9):2363–2370. [PubMed] [Google Scholar]
- Gruenstein E., Rich A., Weihing R. R. Actin associated with membranes from 3T3 mouse fibroblast and HeLa cells. J Cell Biol. 1975 Jan;64(1):223–234. doi: 10.1083/jcb.64.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
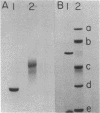
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Herrmann E. C., Moore E. C. Purification of thioredoxin from rat Novikoff ascites hepatoma. J Biol Chem. 1973 Feb 25;248(4):1219–1223. [PubMed] [Google Scholar]
- Holmgren A. Bovine thioredoxin system. Purification of thioredoxin reductase from calf liver and thymus and studies of its function in disulfide reduction. J Biol Chem. 1977 Jul 10;252(13):4600–4606. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent enzyme reactions of the phage T4 ribonucleotide reductase system. J Biol Chem. 1978 Oct 25;253(20):7424–7430. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979 May 10;254(9):3672–3678. [PubMed] [Google Scholar]
- Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Purification and characterization of glutaredoxin from Escherichia coli. J Biol Chem. 1979 May 10;254(9):3664–3671. [PubMed] [Google Scholar]
- Holmgren A., Morgan F. J. Enzyme reduction of disulfide bonds by thioredoxin. The reactivity of disulfide bonds in human choriogonadotropin and its subunits. Eur J Biochem. 1976 Nov 15;70(2):377–383. doi: 10.1111/j.1432-1033.1976.tb11027.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Lara C., de la Torre A., Buchanan B. B. Ferralterin: an iron-sulfur protein functional in enzyme regulation in photosynthesis. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1337–1344. doi: 10.1016/0006-291x(80)90566-5. [DOI] [PubMed] [Google Scholar]
- Lik-Shing Tsang M., Schiff J. A. Properties of enzyme fraction A from Chlorella and copurification of 3' (2'), 5'-biphosphonucleoside 3' (2')-phosphohydrolase, adenosine 5'phosphosulfate sulfohydrolase and adenosine-5'-phosphosulfate cyclase activities. Eur J Biochem. 1976 May 17;65(1):113–121. doi: 10.1111/j.1432-1033.1976.tb10395.x. [DOI] [PubMed] [Google Scholar]
- Luthman M., Eriksson S., Holmgren A., Thelander L. Glutathione-dependent hydrogen donor system for calf thymus ribonucleoside-diphosphate reductase. Proc Natl Acad Sci U S A. 1979 May;76(5):2158–2162. doi: 10.1073/pnas.76.5.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE E. C., REICHARD P., THELANDER L. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES.V. PURIFICATION AND PROPERTIES OF THIOREDOXIN REDUCTASE FROM ESCHERICHIA COLI B. J Biol Chem. 1964 Oct;239:3445–3452. [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigiet V. P., Conley R. R. Purification of thioredoxin, thioredoxin reductase, and glutathione reductase by affinity chromatography. J Biol Chem. 1977 Sep 25;252(18):6367–6372. [PubMed] [Google Scholar]
- Pigiet V., Conley R. R. Isolation and characterization of phosphothioredoxin from Excherichia coli. J Biol Chem. 1978 Mar 25;253(6):1910–1920. [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Thelander L. Thioredoxin reductase. Characterization of a homogenous preparation from Escherichia coli B. J Biol Chem. 1967 Mar 10;242(5):852–859. [PubMed] [Google Scholar]
- Tsang M. L. Assimilatory sulfate reduction in Escherichia coli: identification of the alternate cofactor for adenosine 3'-phosphate 5'-phosphosulfate reductase as glutaredoxin. J Bacteriol. 1981 Jun;146(3):1059–1066. doi: 10.1128/jb.146.3.1059-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. L., Lemieux J., Schiff J. A., Bojarski T. B. Preparation of adenosine 5'-phosphosulfate (APS) from adenosine 3'-phosphate 5'-phosphosulfate (PAPS) prepared by an improved procedure. Anal Biochem. 1976 Aug;74(2):623–626. doi: 10.1016/0003-2697(76)90249-9. [DOI] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Assimilatory sulfate reduction in an Escherichia coli mutant lacking thioredoxin activity. J Bacteriol. 1978 Apr;134(1):131–138. doi: 10.1128/jb.134.1.131-138.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang M. L., Schiff J. A. Sulfate-reducing pathway in Escherichia coli involving bound intermediates. J Bacteriol. 1976 Mar;125(3):923–933. doi: 10.1128/jb.125.3.923-933.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger W., Follmann H. A thioredoxin from green algae. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1044–1051. doi: 10.1016/s0006-291x(77)80083-1. [DOI] [PubMed] [Google Scholar]
- Weatherbee J. A., Luftig R. B., Weihing R. R. In vitro polymerization of microtubules from HeLa cells. J Cell Biol. 1978 Jul;78(1):47–57. doi: 10.1083/jcb.78.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weatherbee J. A., Luftig R. B., Weihing R. R. Purification and reconstitution of HeLa cell microtubules. Biochemistry. 1980 Aug 19;19(17):4116–4123. doi: 10.1021/bi00558a033. [DOI] [PubMed] [Google Scholar]
- Wolosiuk R. A., Crawford N. A., Yee B. C., Buchanan B. B. Isolation of three thioredoxins from spinach leaves. J Biol Chem. 1979 Mar 10;254(5):1627–1632. [PubMed] [Google Scholar]