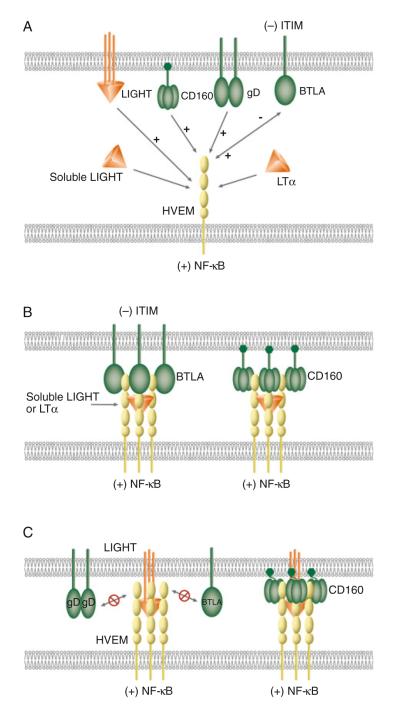
Fig. 36.1.
Schematic illustrations of molecular interactions between HVEM and its ligands. (a) Canonical and unconventional ligands of HVEM. LIGHT and LTα are the canonical ligands as well as positive activators of HVEM. Ligation of LIGHT or LTα activates HVEM-dependent NF-κB signaling. BTLA, CD160, and HSV gD serve as unconventional ligands for HVEM. Ligation of CD160 or gD to HVEM induces NF-κB activation. Bidirectional signaling occurs between HVEM and BTLA. HVEM-mediated BTLA signaling induces tyrosine phosphorylation of the ITIM in BTLA, providing an inhibitory signal to T cells. BTLA, CD160, and gD bind HVEM at the CRD1, but LIGHT and LTα interact with HVEM in the CRD2 and 3. The binding of gD to HVEM blocks HVEM binding to LIGHT or BTLA (not shown in diagram). (b) Cooperative binding between soluble LIGHT, LTα, HVEM, BTLA, and CD160. Left complex shows formation of a LIGHT–HVEM–BTLA trimolecular complex with three molecules of soluble LIGHT, HVEM, and BTLA. Right complex shows the assembling of LIGHT–HVEM–CD160 trimolecular complex with three molecules of soluble LIGHT and HVEM, and nine molecules of CD160. (c) Model of membrane LIGHT, HVEM, BTLA, and CD160 interaction. Membrane LIGHT competes with BTLA for the binding with HVEM. The binding of membrane LIGHT to HVEM prevents BTLA or gD from binding to HVEM due to membrane restriction (left complex). The flexibility of the GPI-link in CD160 may accommodate the membrane restraint for the formation of membrane LIGHT–HVEM–CD160 trimolecular complex (right complex)