Abstract
BACKGROUND AND PURPOSE
Centrally acting histamine H3 receptor ligands are proposed as potential treatments for obesity, although the value of inverse agonists at these receptors is still debated. Functional inhibition of H3 autoreceptors activates neurones in a hypothalamic ‘satiety’ centre. The H3 receptor antagonist, proxyfan was used as a tool to assess the action of histaminergic compounds in this model.
EXPERIMENTAL APPROACH
We compared the actions of histamine on feeding with those of an H3 receptor agonist (imetit) and inverse agonist (thioperamide) in rats and mice. Sites of action were identified by immunohistochemistry and the hypothalamic ventromedial nucleus (VMN) was investigated using electrophysiological techniques.
KEY RESULTS
Central histamine or thioperamide decreased fast-induced feeding, whereas imetit increased feeding. Systemic thioperamide entered the brain to activate hypothalamic feeding centres and to reduce feeding without causing any adverse behaviours. Thioperamide activated neurones in the VMN through an action on histamine autoreceptors, whilst imetit had the opposite effect. Proxyfan administered alone did not affect either feeding or electrical activity. However, it blocked the actions of both thioperamide and imetit, acting as a neutral antagonist in this system.
CONCLUSIONS AND IMPLICATIONS
The H3 receptor inverse agonist, thioperamide, potently reduced appetite without adverse behavioural effects. This action was blocked by proxyfan, acting as a neutral antagonist in this model and, therefore, this compound is useful in determining the selectivity of H3 receptor-directed drugs. A major action of thioperamide is through presynaptic autoreceptors, inducing stimulation by endogenous histamine of postsynaptic H1 receptors on anorectic hypothalamic neurones.
Keywords: histamine, appetite, obesity, proxyfan, thioperamide, ventromedial nucleus
Introduction
Histamine is a multifunctional amine messenger, implicated to function in many body systems. Within the brain it is important in arousal, cognition, the autonomic and neuroendocrine systems, and has a strong influence on ingestive behaviour (Haas et al., 2008). Inhibitors of the synthetic enzyme, histidine decarboxylase, or histamine H1 receptor antagonists increase food consumption (Fukagawa et al., 1989; Orthen-Gambill and Salomon, 1992; Ookuma et al., 1993; Lecklin et al., 1998; receptor nomenclature follows Alexander et al., 2011). By contrast, increasing the availability of endogenous histamine suppresses appetite (Sheiner et al., 1985; Ookuma et al., 1993; Malmlof et al., 2007; Ishizuka et al., 2008). The level of histamine in the hypothalamus increases with appetitive behaviour (Itoh et al., 1991), and the mediobasal region of the hypothalamus has been proposed as a site of action following region-specific injections of histaminergic drugs (Sakata et al., 1988; 1990; Fukagawa et al., 1989). Specifically, the ventromedial nucleus (VMN) of the hypothalamus has long been known as an important ‘satiety’ centre from classic brain-lesioning studies, and to be the region most densely populated with glucose-sensing neurones (King, 2006). Thus, the VMN has important roles in monitoring and responding to both acute and longer-term changes in energy status. It is a major site for the action of the adipose cell-derived hormone, leptin (Dhillon et al., 2006), as well as a key region for countering hypoglycaemia (Borg et al., 1997). Despite its importance in such key homeostatic mechanisms, still relatively little is known about the phenotypes of the neurones involved, although pituitary adenylate cyclase-activating polypeptide (Hawke et al., 2009), brain-derived neurotrophic factor (Unger et al., 2007) and glutamate-containing (Tong et al., 2007) neurones are clearly involved.
Because of the marked effects of histamine and H1 receptors on peripheral targets, including the immune system (Haas et al., 2008), it would be impossible to contemplate compounds acting on H1 receptors that could act selectively only on central circuits involved in body weight or glucose homeostasis. However, because the H3 receptor is expressed predominantly within the CNS, compounds acting on these receptors have been developed with the view to targeting feeding behaviour (Hancock and Brune, 2005; Leurs et al., 2005). The H3 receptor is mainly a presynaptic receptor, expressed both on histaminergic neuronal cell bodies and terminals in many areas of the brain (Lovenberg et al., 1999; Pillot et al., 2002) and also on heterologous synapses (including those containing other amine transmitters (Arrang et al., 1983; Leurs et al., 2005). Importantly, the H3 receptor also shows a high level of constitutive activity, showing that there is a strong endogenous ‘brake’ on the central release of histamine in regions where the receptors are located (Morisset et al., 2000; Arrang et al., 2007). With this discovery, many H3 receptor antagonists were reclassified as inverse agonists capable of negatively regulating adenylate cyclase through binding Gi/o proteins (Arrang et al., 1983; Lovenberg et al., 1999; Wulff et al., 2002). The vast majority of published data indicate that H3 receptor inverse agonists decrease food intake in rats (Itoh et al., 1998; Lecklin and Tuomisto, 1998; Lecklin et al., 1998; Hancock et al., 2005; Malmlof et al., 2005; 2006), mice (Hancock et al., 2004; 2005), hamsters (Jethwa et al., 2009), pigs and primates (Malmlof et al., 2007), whilst agonists increase feeding (Kent et al., 1997; Chiba et al., 2009; Jethwa et al., 2009). However, there are other papers that have found varying efficacy (Merali and Banks, 1994; Itoh et al., 1998; Sindelar et al., 2004), and one paper that found opposing effects with compounds acting on H3 receptors (Yoshimoto et al., 2006).
An exact understanding of how histaminergic drugs act in hypothalamic feeding circuits has been hampered by the lack of good neutral antagonists. Proxyfan is classified as an antagonist with a Ki of 1–5 nM at H3 receptors which is more than 1000-fold lower than those for other histamine receptors (Ligneau et al., 1994; Morisset et al., 2000; Gbahou et al., 2003). However, as with a number of G protein-coupled receptors, H3 receptors can exhibit different levels of constitutive activity in vivo and proxyfan has the potential to act pharmacologically through a spectrum of potencies (Arrang et al., 2007). As constitutive activity does vary, it is important to determine the specific actions of H3 receptor ligands in the system that is being manipulated. Proxyfan has been used relatively infrequently in whole animal studies. It was a H3 receptor agonist in contextual fear memory formation (Baldi et al., 2005), a potential inverse agonist in arousal and glucose handling (Gbahou et al., 2003; Henry et al., 2011) or a neutral antagonist in drinking behaviour (Fox et al., 2002). Here, using the rat as our model, we showed, in vivo, that the VMN is a major target for compounds acting on histamine receptors that affect feeding behaviour. In vitro electrophysiology demonstrates the sensitivity of the majority of neurones in the dorsomedial region of the VMN to an excitatory action of histamine through H1 receptors and that this pathway can be modulated locally by presynaptic H3 autoreceptors. We show that proxyfan acts as a neutral antagonist in this system, blocking the actions of both H3 receptor agonists and inverse agonists. Furthermore, proxyfan has the same effects in vivo to block the actions of other compounds acting on H3 receptors, without affecting feeding behaviour itself.
Methods
Animals and feeding experiments
All animal care and experimental procedures complied with the Home Office (Animals) Procedures Act (1986) and were approved after local ethical review. The results of all studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010).
Outbred, male Sprague-Dawley rats (225–275 g) or CD1 mice (25–30 g; both from Charles River, Margate, UK) were used (total = 290). Animals were kept in a 12 h light/12 h dark cycle (lights on 0800–2000) within the University of Manchester animal facility for at least 1 week prior to the start of each experiment. The animals were housed in a temperature-controlled room (22 ± 1°C) with a relative air humidity of 40–60%, and with free access to food (Beekay, Hull, UK) and water. Animals were housed singly to allow measurement of normal, night-time food intake, or overnight fast-induced re-feeding. Drugs were administered either i.p. (volumes of 1 mL·kg−1 body weight in isotonic saline) or i.c.v. (2 µL per animal). Immediately following drug administration, weighed food was presented and intake measured at 1, 2, 4, 12 and 24 h later.
For the placement of in-dwelling i.c.v. cannulae, rats were anaesthetized with 2% isofluorane (Concord Pharmaceuticals Ltd, Reading, UK) in O2 at 1 L·min−1. A 21-gauge guide cannula was implanted 0.8 mm posterior and 1.5 mm lateral to bregma, and 3 mm into the brain to access the lateral ventricle (Paxinos and Watson, 1986). This was fixed to the skull with acrylic dental cement (Simplex Rapide, Austenal Dental, Hoorn, the Netherlands) adhered to two jeweller's screws. For post-operative analgesia, rats were injected with 10 µL·kg−1 buprenophrine (Vetergesic, Reckitt Benckiser Healthcare, Hull, UK) and a bolus of saline to aid recovery, and left for 1 week before experimentation.
To measure the behavioural satiety sequence, rats (n= 8) were housed singly, 2 days before and then throughout the experiment, in transparent observation cages. Treatments were randomized. Food was removed 2 h before the experiment began to ensure no pre-feeding occurred. Each animal received one injection of each treatment with a minimum of 3 days between each experiment. After the drug was administered, rats were given a pre-weighed amount of food and behavioural observations began 90 s later. The animals were scored every 30 s over a 90-min period, with the behaviour recorded as either feeding, drinking, grooming, resting, inactive or active (Dodd et al., 2010). The data were collated into 5-min period bins and expressed as the mean percentage of total behaviour for each animal and then for the treatment. In addition, the amount of food eaten by each animal over the 90-min observational period was recorded.
Functional immunohistochemistry
Ninety minutes following injection, the rats were deeply anaesthetized with sodium pentobarbitone (100 mg·kg−1; B. Braun, Sheffield, UK) and perfused transcardially with heparinized (10 000 i.u.·L−1; Leo Pharma, Ballerup, Denmark) isotonic saline (0.9% NaCl) for 8 min, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.0) for 15 min. Perfused brains were post-fixed and cryoprotected in the same fixative with 15% sucrose added, followed by immersion in 30% sucrose in 0.1 M phosphate buffer. Using a freezing-sledge microtome, 30 µm coronal sections throughout the rostro-caudal extent of the brain were cut. Endogenous peroxidase activity was deactivated by incubating sections in a 1.5% hydrogen peroxide, 20% methanol and 0.2% Triton X-100 in 0.1 M phosphate buffer for 30 min at room temperature, followed by washes in 0.1 M phosphate buffer. To block non-specific staining, slices were incubated for 1 h at room temperature in 2% normal goat serum (2% NGS, 0.1 M phosphate buffer, 0.3% Triton X-100). Slices were then incubated for ∼24 h at 4°C in goat anti-rabbit c-Fos antibody (SC052, Santa Cruz Biotechnology Inc., Santa Cruz, USA) diluted 1:1000 in the NGS blocking serum, then sequentially in goat anti-rabbit immunoglobulin-peroxidase complex (Vector Laboratories, Peterborough, UK) diluted 1:500 in blocking serum followed by streptavidin-biotin-horseradish peroxidase complex diluted 1:400 in 0.1 M phosphate buffer. Staining was visualized using nickel-intensified diaminobenzidine (Vector Laboratories). Neurones were determined to be c-Fos-positive if their nuclei were stained a dark black colour. A qualitative analysis of the whole brain was made to determine regions of interest (i.e. that contained significant c-Fos staining). Regions of interest were then counted bilaterally for each animal (4–8 sections) with the observer unaware of the treatment group. The average number of cells per section was calculated for each animal and further averaged to provide a treatment group mean.
Extracellular electrophysiology
Rats were killed by cervical dislocation and decapitation under isofluorane anaesthesia. The brain was rapidly removed and dissected to form a tissue block containing the hypothalamus. Coronal brain slices (400 µm thick) were cut in ice-cold, artificial cerebrospinal fluid (aCSF; NaCl, 124.0 mM; NaHCO3, 25.5 mM; KCl, 3.3 mM; KH2PO4, 1.2 mM; MgSO4, 1.0; CaCl2, 2.5 mM; d-glucose, 5 mM; adjusted to pH 7.4 and constantly perifused with 95% O2/5% CO2) using a Vibroslicer (Campden Instruments, Loughborough, UK). Brain slices were transferred to a PDMI-2 submerged slice microincubator (Medical Systems Corp., New York, NY, USA) and maintained for 8–12 h by perifusion (approximately 1.5 mL·min−1) with oxygenated aCSF. The tissue bath and perfusion solutions were warmed to approximately 35°C using a TC-202 temperature controller (Medical Systems Corp.). Slices were allowed to equilibrate for at least 1 h before electrophysiological recordings began. Single-unit activity of hypothalamic neurones was recorded extracellularly with borosilicate glass electrodes (Harvard Instruments, Herts, UK) filled with 2 M NaCl (resistance approximately 5 MΩ). Action potential spikes were amplified (×20 000) and filtered (bandwidth 300 Hz to 3 kHz). Amplification, filtering and spike discrimination were performed using a NeuroLog modular system (Digitimer Ltd., Welwyn Garden City, UK). Data were collected and plotted as integrated histograms using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). The size of the spikes recorded was between 10 and 100 mV. Only spikes at least two times the size of the background noise were recorded and the cell firing rate was recorded for at least 10 min to obtain a stable baseline firing rate, prior to a change in aCSF or drug treatment. Recordings were only taken from the neurones in the dorsomedial region of the VMN. A VMN neurone was considered to have responded if the firing rate increased or decreased by 20% relative to the previous 5 min baseline recording.
Data analysis
Data on food intake are presented as means ± SEM, and were analysed using an unpaired t-test or by one-way anova with post hoc Tukey's multiple comparison tests. We also present the total time spent in each behaviour during the BSS in Supporting Information Table S1, and analysed this using a non-parametric Kruskall–Wallis test with Dunn's post hoc comparisons. Results from the c-Fos immunochemistry were derived from each region of interest and means (± SEM) from each region were compared independently using a Mann–Whitney U-test. Electrophysiological responses in VMN neurones are shown as mean firing rates (± SEM) and compared with an unpaired t-test. In all experiments, significance was taken at P < 0.05.
Materials
All chemicals (including histamine dihydrochlroide, thioperamide maleate, imetit dihydrobromide, pyrilamine maleate) were supplied by Sigma-Aldrich Company Ltd. (Poole, UK), unless stated otherwise. Proxyfan oxalate was bought from Tocris Bioscience (Bristol, UK).
Results
Compounds acting on histamine receptors affect feeding without disrupting other aspects of normal behaviour
Although the effect was relatively transient and food intake had normalized by 24 h, 200 nM histamine i.c.v. potently decreased fast-induced re-feeding (Figure 1). The effect of centrally administered histamine was compared with that of two highly selective H3 receptor ligands: the agonist, imetit, which has a Ki of 0.1–1 nM and the inverse agonist, thioperamide, which has a Ki of 1–5 nM at H3 receptors, both with negligible affinity for other histamine and non-histamine receptors (Arrang et al., 1987; Garbarg et al., 1992). Imetit caused a dose-dependent increase in food intake in the first hour following injection when administered i.c.v. (Figure 2). The anorexigenic effect of thioperamide though noticeable earlier, only became significant statistically at the 4 h measurement when injected centrally. The two H3 receptor-selective drugs were also effective when administered i.p., suggesting that they crossed the blood-brain barrier to have their action on feeding (Figure 2). Respectively, imetit (10 mg·kg−1) and thioperamide (2 mg·kg−1) produced strong orexigenic and anorexigenic effects, and were chosen to examine the behavioural satiety sequence more closely.
Figure 1.
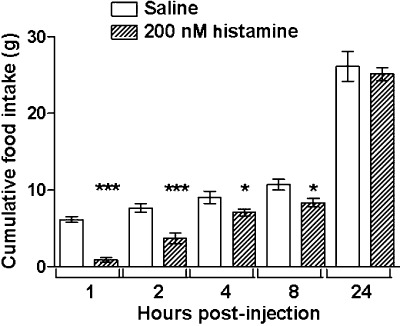
Food consumption measured at 1, 2, 4, 8 and 24 h after i.c.v. injection of histamine (200 nM, n= 11 per group) or control saline (n= 9). *P < 0.05, ***P < 0.005, significantly different from values with saline; unpaired t-test.
Figure 2.
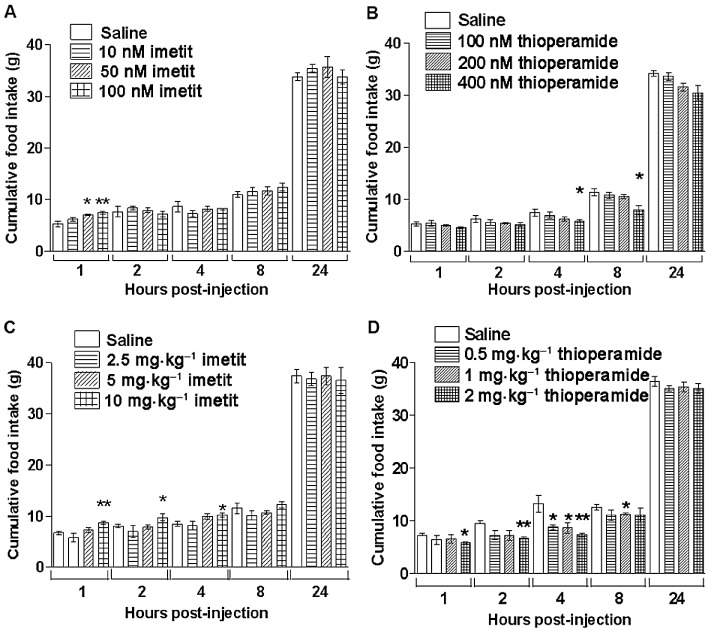
Food consumption measured at 1, 2, 4, 8 and 24 h after i.c.v. injection with (A) imetit (0, 10, 50 and 100 nM; n= 6–7 per group) or (B) thioperamide (0, 100, 200 and 400 nM, n= 5). In separate experiments, food consumption was measured after i.p. injection with (C) imetit (0, 2.5, 5 and 10 mg·kg−1; n= 6–7) or (D) thioperamide (0, 0.5, 1 and 2 mg·kg−1; n= 5), *P < 0.05, **P < 0.01, significantly different from corresponding values with saline; one-way anova with Tukey's multiple comparison post hoc test.
In the next experiment, each rat received three treatments with at least 3 days in between. Injections were given i.p. just after lights out, and normal night-time feeding behaviour recorded for the subsequent 90 min. Over this period, the drugs had the expected effects on food intake (average amount of chow eaten over 90 min: vehicle 6.01 g, imetit 7.24 g, thioperamide 4.39 g, both P < 0.05 compared with vehicle-treated group). The overall pattern of the behavioural satiety sequence was not disrupted by either treatment, suggesting the drugs were not having any adverse effects, such as causing nausea or sedation (Figure 3). Rats injected with imetit spent 34 ± 3% of their time eating, whereas control animals spent 31 ± 3% (not significant; Supporting Information Table S1). All times spent in other behaviours corresponded with those of control animals. Thioperamide-treated rats also showed behaviours similar to those of control rats and spent a similar percentage of time in each behaviour. The one behaviour to deviate from control levels was the time spent feeding. Thioperamide-treated animals spent 24 ± 3% of their time feeding over the 90 min they were observed, although this did not quite reach statistical significance (Supporting Information Table S1).
Figure 3.
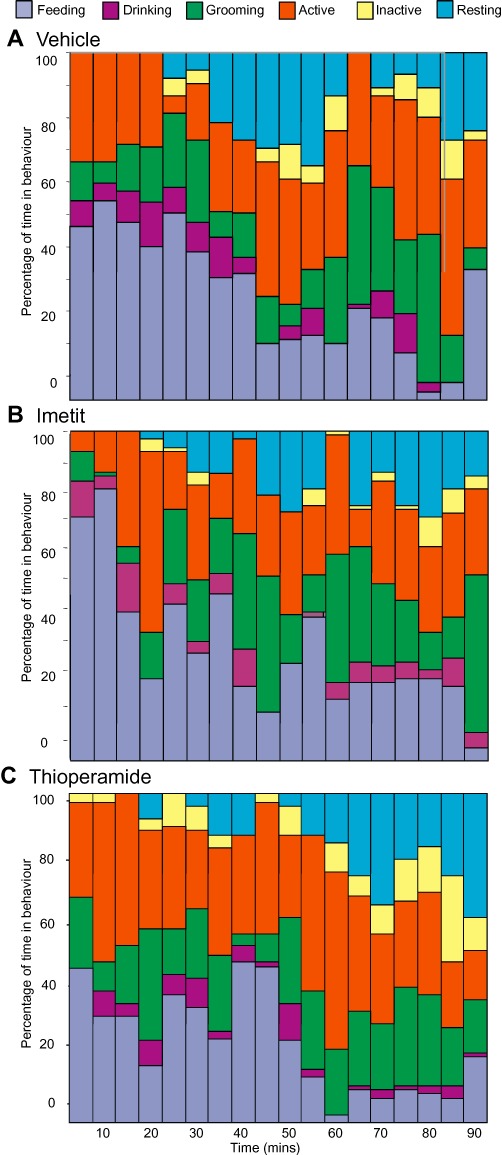
Behavioural satiety sequence. Rats (n= 8) were injected i.p. with (A) control saline, (B) imetit or (C) thioperamide, and introduced to food. Their behaviours were scored every 30 s for a period of 90 min according to the categories mentioned in the method section. Data were collated into 5 min time bins and expressed as mean percentage of total behaviour for each animal and then for the treatment. A comparison of percentage time spent in each behaviour over the whole 90 min period is shown in Supporting Information Table S1.
Yoshimoto et al. (2006) found that in mice, thioperamide (3–30 mg·kg−1) given orally caused an increase in food intake which, although by a different route of administration, might suggest a species difference. Thus, we examined the effect of thioperamide (10–20 mg·kg−1, i.p.) in mice at lights off. Thioperamide caused a dose-dependent decrease in night-time food intake (vehicle 0.75 g; 10 mg kg-1 thioperamide 0.51 g, P < 0.001; 20 mg·kg−1 thioperamide 0.27 g, P < 0.01; compared with vehicle group) in adult, outbred mice.
Anorectic doses of histamine and thioperamide activate hypothalamic nuclei
Thioperamide enters the brain, where most H3 receptors are located, to reduce food intake. In order to determine whether thioperamide was having widespread effects in the brain, we compared the ability of anorectic doses of i.c.v. histamine and i.p. thioperamide to induce expression of the functional activity marker, c-Fos. Histamine (200 nM, i.c.v.) did not cause global activation of the brain, but instead, activity was distributed within clearly identifiable regions (Figure 4). The staining pattern of c-Fos was similar following administration of thioperamide (2 mg·kg−1, i.p.), and therefore, we focused on these regions of interest in our quantitative analyses. The regions of interest were the VMN, dorsomedial nucleus (DMN), arcuate nucleus (ARC) and paraventricular nucleus (PVN) in the hypothalamus, anterior hypothalamic area, lateral hypothalamic area (LHA), medial amygdala, anterior amygdala, central amygdala, tuberomammillary nucleus and the dorsal raphe nucleus. These regions of interest were counted, and most regions displayed a significant increase in the number of c-Fos-positive cells per section following treatment with histamine (Figure 5) or thioperamide (Supporting Information Figure S1). Although values for the VMN cover this whole anatomical nucleus, it is clear that activation is mainly in the dorsomedial part of the VMN, the region linked closest with the regulation of feeding (Hawke et al., 2009). Therefore, we concentrated on this specific region when making electrophysiological recordings.
Figure 4.
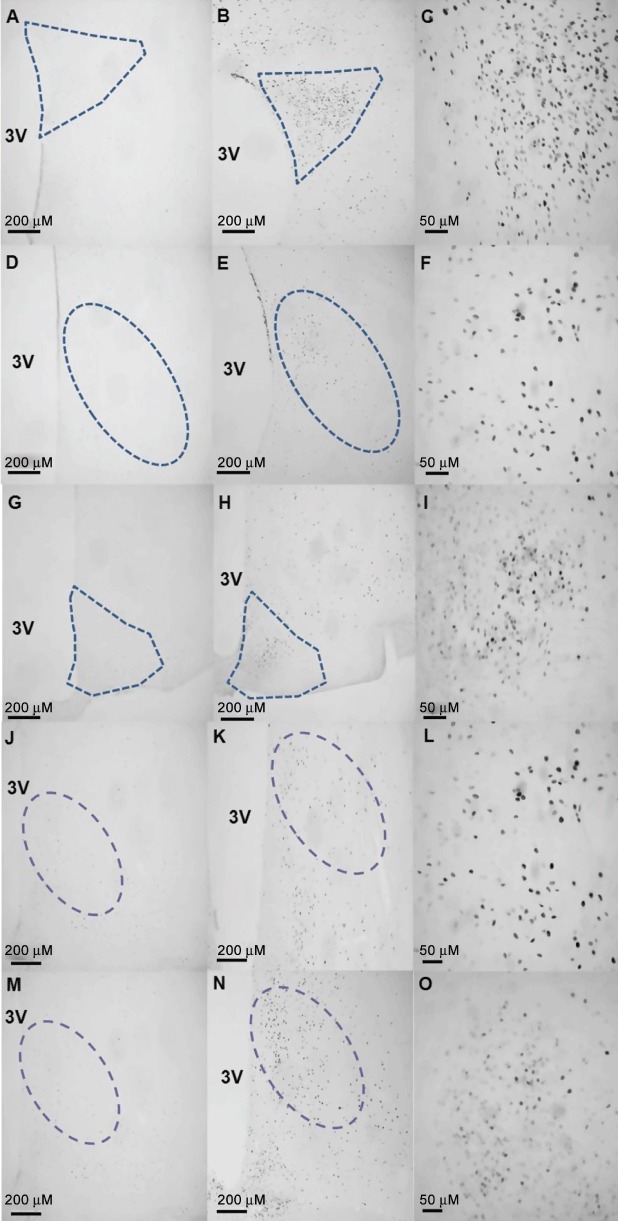
Representative photomicrographs of examples of regions of interest shown to exhibit a significantly high level of activation following histamine administration. Rats received an injection of control saline (A, D, G, J, M), histamine (200 nM; B, E, H), imetit (10 mg·kg−1; K) or thioperamide (2 mg·kg−1; N) into the cerebral ventricle 90 min before transcardial perfusion with fixative. Histamine is seen to induce the expression of c-Fos in the paraventricular (B, C), ventromedial (E, F) and arcuate (H, I) nuclei of the hypothalamus. Also shown, is c-Fos expression in the ventromedial nucleus following administration of imetit (K, L) or thioperamide (N, O).
Figure 5.
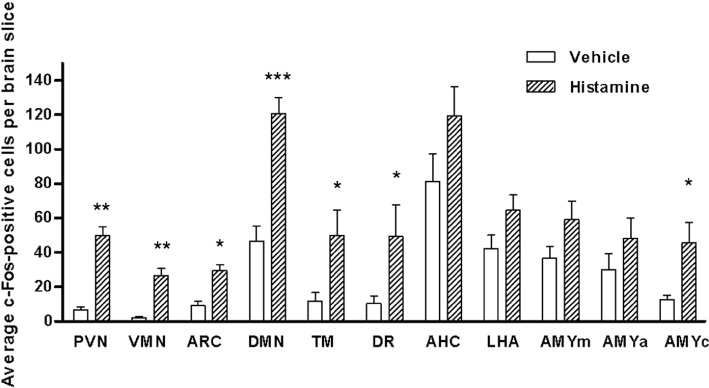
Quantification of the induction of c-Fos in regions of interest following administration of vehicle or 200 nM histamine (i.c.v.). The bars represent means and SEM of the number of Fos-positive nuclei per section. Four to eight sections were counted for each region in each animal. PVN, paraventricular nucleus; VMN, ventromedial nucleus; ARC, arcuate nucleus; DMN, dorsomedial nucleus; TM, tuberomammillary nucleus; DR, dorsal raphe nucleus; AHC, anterior hypothalamic area; LHA, lateral hypothalamic area; AMYm, medial amygdala; AMYa, anterior amygdala; AMYc, central amygdala. *P < 0.05; **P= 0.01; ***P= 0.001, significantly different from values with vehicle; Mann–Whitney U-test.
Electrophysiological interactions between histamine H3 and H1 receptors in the dorsomedial VMN
A total of 197 spontaneously firing neurones in the dorsomedial region of the VMN were tested for their responsiveness to bath application of histamine (5 µM). The cells showed an average basal firing rate of 1.91 ± 1.73 Hz and a range of 0.97–3.55 Hz. 122 out of 197 cells (62%) showed an increased firing when histamine was applied, whilst four decreased firing (2%) and 71 had no response (36%). Those that increased firing had an average rate of 3.47 ± 2.01 Hz following application of histamine. It is important to note that extracellular electrophysiology is biased towards recording from spontaneously active cells. Thus, the proportion of cells in each category is only an estimate, and more silent cells may be recruited by histamine than this estimation predicts. Thirty-one VMN neurones that were responsive to the application of histamine were then tested by the co-application of histamine (5 µM) and pyrilamine (50 µM), an H1 receptor antagonist. In the majority of cells, pyrilamine blocked the effects of histamine (28 out of 31 cells, 90%; Figure 6A). All changes in neuronal activity were shown to return to basal levels within 20 min after application of either drug.
Figure 6.
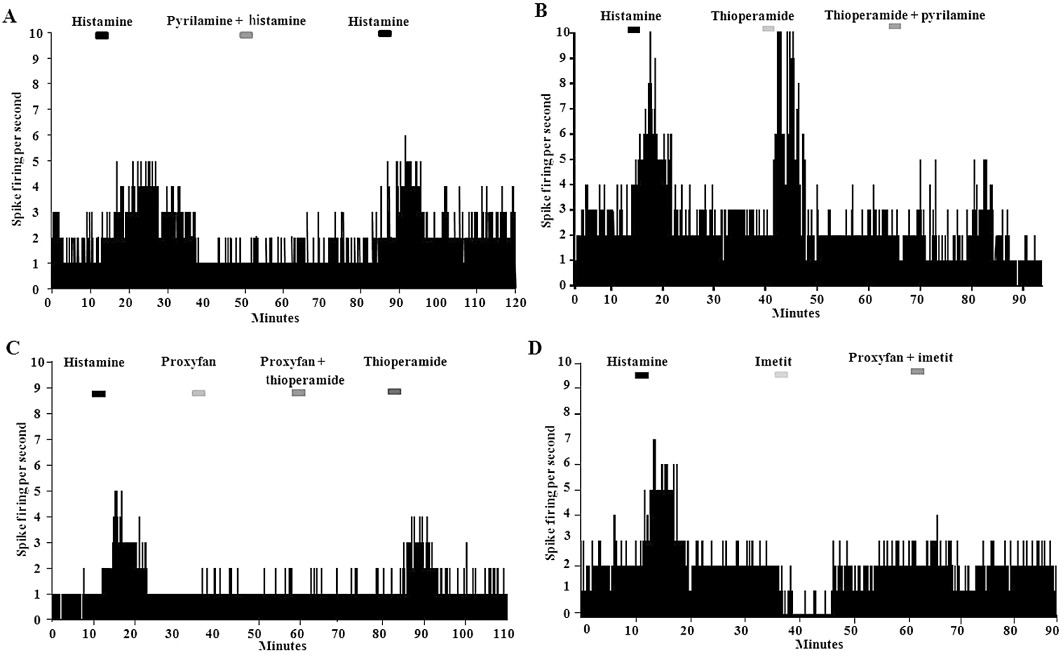
(A) Trace showing typical responses of a VMN neurone to the application of 5 µM histamine alone or with co-administration of pyrilamine (an H1 receptor antagonist; 50 µM) using extracellular electrophysiology in a rat brain slice. Blocks at the top of the diagram indicate when the treatments were perifused in the bath (5 min applications). The Y-axis represents the firing rate of the neurone (spikes per second). A stable baseline was re-established between drug administrations. (B) Recording from another histamine-responsive neurone, showing increased firing when thioperamide (20 µM) is applied. The effect of thioperamide is also blocked by pyrilamine. (C) VMN neurones do not respond to proxyfan when administered alone (20 µM). However, proxyfan can block the activation caused by thioperamide. (D) 20 µM imetit (20 µM), the H3 receptor agonist, causes an inhibition of histamine-responsive VMN neurones. This effect is also antagonized by proxyfan.
Forty-nine histamine-responsive VMN neurones were tested for their reaction to the application of the H3 receptor inverse agonist, thioperamide (20 µM) alone. Forty-three out of 49 cells (88%) showed an increase in firing when thioperamide was applied (average firing rate of 3.15 ± 2.01 Hz), whilst one cell decreased firing and five had no response. Further, we tested 15 histamine-responsive VMN neurones with 20 µM thioperamide applied alone and then co-applied with 50 µm pyrilamine. All 15 cells showed increases in neuronal firing with thioperamide alone, but this was blocked when pyrilamine was co-applied with the thioperamide (Figure 6B).
Twenty spontaneous (average basal firing rate of 1.86 ± 1.33 Hz), histamine-responsive VMN neurones were tested, but none responded to the application of 20 µM proxyfan with a change in firing rate. However, this same dose of proxyfan was able to block the excitatory effects of thioperamide (Figure 6C). A further 22 cells were tested with the H3 receptor agonist, imetit, alone. Of these, 19 (86%) showed a reduction in firing rate when imetit (5 µM) was applied (average firing rate reduced from 1.86 ± 1.33 Hz to 0.47 ± 0.42 Hz). In 100% of cells tested (20/20), proxyfan blocked the inhibitory effect of imetit on firing (Figure 6D).
Proxyfan antagonizes the actions of both thioperamide and imetit on feeding
Proxyfan is capable of a spectrum of potencies depending on the level of spontaneous receptor activity. We had shown that, in our in vitro model, proxyfan acts as a neutral antagonist, so we now tested whether this was true also within an in vivo model of feeding. Levels of endogenous histamine in the whole brain may vary greatly across the time of day, and also in regions of the brain specifically involved in appetite regulation. Thus, we measured the effects of proxyfan alone at both lights out and at lights on, in rats that were fed ad libitum, ensuring a spectrum of endogenous histamine concentrations would be tested. Proxyfan injected i.p. in a range of doses (0.2–5.0 mg·kg−1 body weight) had no effect on food intake in normally satiated animals during the day time, or in night-time feeding animals (Supporting Information Figure S2).
In two separate experiments using normal feeding rats during the night time, proxyfan (5 mg·kg−1) blocked the orexigenic action of the H3 receptor agonist, imetit (10 mg·kg−1, i.p.) and the anorectic action of the H3 receptor inverse agonist, thioperamide (2 mg·kg−1, i.p.; Figure 7).
Figure 7.
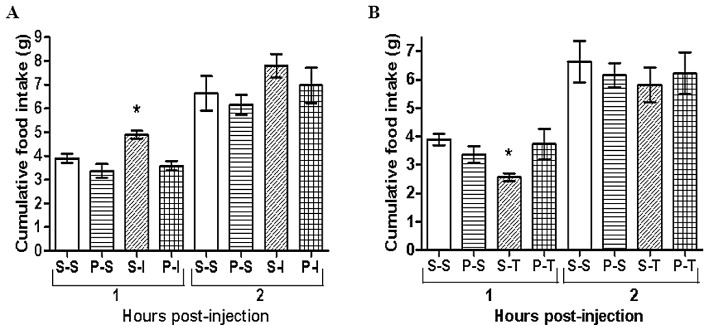
(A) Cumulative food consumption measured at 1 and 2 h after two i.p. injections with control saline (S-S), proxyfan (5 mg·kg−1) followed by saline (P-S), saline followed by imetit (10 mg·kg−1; S-I), or proxyfan followed by imetit (P-I). (B) In a separate experiment, injections were saline-saline (S-S), proxyfan-saline (P-S), saline-thioperamide (2 mg·kg−1; S-T), proxyfan-thioperamide (P-T). n= 6 in all groups; the two injections were given 15 min apart. P < 0.05; one-way anova with Tukey's multiple comparison post hoc test.
Discussion
The effect of histamine in the CNS is anorexigenic and, therefore, overcoming the activity of presynaptic H3 autoreceptors is hypothesized to increase the release of endogenous histamine and to reduce food intake (Hancock and Brune, 2005; Leurs et al., 2005). However, three papers cast doubt on this theory. Firstly, Takahashi et al. (2002) reported that the H3 receptor knockout mouse is mildly obese, which opposes what might be expected in the simplest model of a single population of receptor being involved in body-weight regulation. Secondly, in a study of dispersed neurones from the mediobasal hypothalamus, there was an H3 receptor-mediated effect of histamine on GABA-dependent inhibitory postsynaptic potentials (Jang et al., 2001). This would suggest that the H3 receptors in this region may be heteroreceptors rather than presynaptic autoreceptors. Thirdly, Yoshimoto et al. (2006) suggested opposite effects of compounds acting on H3 receptors when given orally, to those characterized in the main body of literature (Kent et al., 1997; Itoh et al., 1998; Lecklin and Tuomisto, 1998; Lecklin et al., 1998; Hancock et al., 2004; 2005; Malmlof et al., 2005; 2006; 2007; Chiba et al., 2009; Jethwa et al., 2009).
Interpreting the phenotype of a receptor knockout animal in isolation can be problematic, and the authors who report that the H3R knockout mice are only mildly obese, do point out that this may be due to up-regulation of other receptor systems in the mouse during development (Takahashi et al., 2002). However, there are other explanations for the phenotype of the mouse which are dependent on the fact that histamine probably affects a wide variety of neurones, some of which might have opposing actions. For example, Fukagawa et al. (1989) reported that H1 receptor antagonism decreased firing in ‘glucose-excited’ neurones of the VMN, but increased firing in ‘glucose-inhibited’ neurones of the LHA. Unfortunately, in this classic paper, the authors did not measure histaminergic actions on glucose-inhibited neurones in the VMN.
In our experiments, we have first shown that the anorectic effects of central histamine or the H3 receptor inverse agonist, thioperamide, are associated with an increase in neuronal activity in the VMN, as measured in normally behaving animals. The behavioural satiety sequence was maintained when we administered thioperamide, suggesting that it reduces feeding without causing any adverse events, although we were unable to study all potential behavioural effects by this method. We then demonstrated in vitro that the excitatory effect of thioperamide on histamine-sensitive neurones in the VMN was blocked in each instance tested by co-administration of the H1 receptor antagonist, pyrilamine. This is novel evidence that H3 receptors are presynaptic autoreceptors located on histaminergic terminals in the VMN, tonically inhibiting the release of endogenous histamine. However, it is not absolute proof and, ideally, we would need to follow this up with patch-clamp recordings to confirm a reduction in histamine-mediated, excitatory postsynaptic potentials. Also, it does not rule out additional possible cellular locations of H3 receptors. Jang et al. (2001) have used a preparation of dispersed hypothalamic neurones to demonstrate that H3 receptor agonists can reduce inhibitory postsynaptic potentials. This would suggest H3 receptors may be present on inhibitory GABAergic terminals and might reduce the release of GABA locally. The VMN is surrounded by GABAergic neurones, which project into the VMN (King, 2006). However, a caveat of this work is that the dispersed neurones are probably from a large area of the mediobasal hypothalamus, rather than from the VMN alone (Jang et al., 2001). The other detailed analysis of the effects of histamine on the electrical activity of VMN neurones comes from the laboratory of Pfaff and colleagues, who are interested in the arousing effects of histamine, and possible interactions with oestrogen, in the regulation of lordosis behaviour (Kow et al., 2005; Zhou et al., 2007; Dupre et al., 2010). Their results show consistently a stimulatory effect of histamine through H1 receptors, but very little effect through H3 receptors. Despite the latter, they find strong expression of H3 receptor message in individually harvested neurones, which could suggest a postsynaptic effect in the VMN. However, it is important to note that their recordings concentrate on the ventrolateral region of the VMN which has the strongest expression of oestrogen receptors and that this region is thought to be more functionally linked with female sexual behaviour rather than appetitive behaviour.
The results of Yoshimoto et al. (2006) are much more difficult to explain, as they found the opposite effect of compounds acting on H3 receptors. For example, they report oral dosing with inverse agonist, thioperamide, increased food intake acutely and body weight over a period. Could there be relevant species differences between mice and rats? This seems unlikely, as there is previously published data showing that H3 receptor inverse agonists reduce feeding in mice (Hancock et al., 2004; 2005), rats (Itoh et al., 1998; Lecklin and Tuomisto, 1998; Lecklin et al., 1998; Hancock et al., 2005; Malmlof et al., 2005; 2006), hamsters (Jethwa et al., 2009), pigs and primates (Malmlof et al., 2007). Indeed, in a small experiment in the current paper, we too found a dose-dependent decrease in feeding with thioperamide. It is noteworthy also that, in a more recent publication from the Yoshimoto group, thioperamide inhibited neuropeptide Y- and nociceptin-induced hyperphagia (Yoshimoto et al., 2008).
The original paper from Yoshimoto et al. (2006) found that the effects of H3 receptor ligands were not apparent in H3 receptor knockout mice. However, as stated previously, any knockout mouse may show compensatory developmental traits, so the use of a neutral antagonist might provide more valuable results. Proxyfan is reported to act as an antagonist at H3 receptors, but it has the potential to act pharmacologically through a spectrum of potencies depending on the level of constitutive receptor activity (Gbahou et al., 2003; Arrang et al., 2007). In our in vitro electrophysiological preparation, we showed that, by itself, proxyfan had no effect on the electrical activity of VMN neurones, yet it could block the actions of both an H3 receptor agonist and an inverse agonist. This suggests that in this model, proxyfan is acting as a neutral antagonist. Likewise, in both fed and fasted rats, proxyfan had no effect itself on feeding, but blocked the anorectic and orexigenic actions of thioperamide and imetit respectively. Thus, proxyfan appears to act as a neutral antagonist in in vivo models of feeding and, as previously published, drinking behaviours (Fox et al., 2002). Thus, proxyfan will be a useful tool in the development of new drugs that target H3 receptors involved in appetite and body-weight regulation.
Concerning the site and mode of action of compounds acting on H3 receptors, we have concentrated on the dorsomedial region of the hypothalamic VMN. Functional activity marking with the immediate-early gene product, c-Fos, demonstrated the activation of this region by anorectic doses of both central histamine and systemic thioperamide. Although such methods will not determine whether the actions are direct, our subsequent electrophysiological recordings would suggest that this is the case, but would need to be proven absolutely by patch-clamp recordings. Other regions of the brain may be as important, and we noted clear activation also in the ARC, DMN and PVN of the hypothalamus. Interpretation is difficult as each of these areas is interconnected and might thus be driven transynaptically. Our electrophysiological data provide evidence for a direct effect at least in the VMN. Sakata et al. (1990) found that injections of a histamine synthesis inhibitor into either the VMN or the PVN elicited feeding, but it was ineffective in other hypothalamic regions. More recently, Barrett et al. (2005) have shown a season-dependent increase in electrical activity within the posterior dorsomedial region of the ARC in Siberian hamsters, which corresponds with a decrease in the expression of H3 receptors in this area. They suggest a role for histamine and, in particular regulation of H3 receptors, in seasonal control of body weight, but which might also be of important consequence to other species. Finally, Poole et al. (2008) found effects of histamine on neurones in the brainstem dorsal vagal complex, suggesting a potential modulatory role in brain-gut signalling. However, these powerful actions on individual neurones were not modified by H3 receptor agonists. Furthermore, in our current experiments, we did not find an obvious effect of either thioperamide or imetit on the rate of satiation (the behavioural satiety sequence was maintained, but not shifted as might be expected for a satiety factor (Lawrence et al., 2002; Scott et al., 2005). Again, this fits with current hypotheses, that histamine is involved in the appetitive phase of feeding, rather than the consummatory phase (Passani et al., 2011).
In conclusion, we provide strong pharmacological, anatomical and electrophysiological evidence that histamine has a major effect on neurones of the VMN, leading to a decrease in food intake. The effect is mediated by H1 receptors on, as yet unidentified neurones in the dorsomedial region of the VMN and is modulated locally by H3 receptors. This region contains the densest population of leptin-sensitive neurones in the VMN, which may contain the peptide, pituitary adenylate cyclase-activating polypeptide as a transmitter (Hawke et al., 2009). Although there may be different locations of H3 receptors, our evidence suggests that they are mainly presynaptic autoreceptors. Despite some recent controversies, H3 receptors are still possible targets for the treatment of over-eating, although it will be necessary to continue the development of selective and brain-penetrating drugs. This search will be facilitated with our finding that, in this system, proxyfan acts as a neutral antagonist both in vitro and in vivo.
Acknowledgments
The authors wish to acknowledge a financial contribution to this work from Novo Nordisk as part of the BBSRC CASE studentship to RHC, and also the technical assistance of Vicki Scott, Hugh Piggins and Stella Macharia.
Glossary
- aCSF
artificial cerebrospinal fluid
- ARC
arcuate nucleus
- DMN
dorsomedial nucleus
- LHA
lateral hypothalamic area
- NGS
normal goat serum
- PVN
paraventricular nucleus
- VMN
ventromedial nucleus of the hypothalamus
Conflicts of interest
This work was financed in part by a supplementation by Novo Nordisk to RHC's PhD studentship. There are no other conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Quantification of the induction ofc-Fos in regions of interest following administration of vehicle orthioperamide (2 mg·kg−1, i.p.). The barsrepresent the number of Fos-positive nuclei per section (mean± SEM). Groups were analysed using a Mann–WhitneyU-test. *P < 0.05;**P = 0.005. VMN, ventromedial nucleus; ARC, arcuate nucleus; DMN, dorsomedial nucleus; TM, tuberomammillary nucleus; DR, dorsal raphe nucleus; AHC, anterior hypothalamic area; AMYm, medial amygdala; AMYa, anterior amygdala; AMYc, central amygdala.
Figure S2 (A) Cumulative food consumptionmeasured at 1, 2 and 4 h after i.p. injection with control salinevehicle (V) or increasing doses of proxyfan (0.2–5.0mg·kg−1). Data are from animals during theday time, when they are normally asleep. (B) In a separateexperiment, injections were at night-time, when the animals areawake and feeding as normal. n = 6 in all groups. No significant effect of proxyfan by itself was recorded.
Table S1 The overall percentage of time spentin each behaviour over the 90-min observational period whilstcarrying out the behavioural satiety sequence and after animalswere injected with saline, imetit or thioperamide. Behaviours werecategorized as follows: feeding, drinking, grooming active,inactive and resting. Data are means ± SEM. Groups wereanalysed using a Kruskall–Wallis test. Significant differencewas seen in inactivity scores between thioperamide and imetit, butnot the control, groups only. *P < 0.05(Dunn's post hoc test).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edn. Br. J. Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Schwartz JC. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Garbarg M, Lancelot JC, Lecomte JM, Pollard H, Robba M, et al. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- Arrang JM, Morisset S, Gbahou F. Constitutive activity of the histamine H3 receptor. Trends Pharmacol Sci. 2007;28:350–357. doi: 10.1016/j.tips.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C, Schunack W, Cenni G, Blandina P, Passani MB. The H3 receptor protean agonist proxyfan enhances the expression of fear memory in the rat. Neuropharmacology. 2005;48:246–251. doi: 10.1016/j.neuropharm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ross AW, Balik A, Littlewood PA, Mercer JG, Moar KM, et al. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinology. 2005;146:1930–1939. doi: 10.1210/en.2004-1452. [DOI] [PubMed] [Google Scholar]
- Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI. Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycemia in awake rats. J Clin Invest. 1997;99:361–365. doi: 10.1172/JCI119165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S, Itateyama E, Sakata T, Yoshimatsu H. Acute central administration of immepip, a histamine H3 receptor agonist, suppresses hypothalamic histamine release and elicits feeding behavior in rats. Brain Res Bull. 2009;79:37–40. doi: 10.1016/j.brainresbull.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Dodd GT, Mancini G, Lutz B, Luckman SM. The peptide hemopressin acts through CB1 cannabinoid receptors to reduce food intake in rats and mice. J Neurosci. 2010;30:7369–7376. doi: 10.1523/JNEUROSCI.5455-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre C, Lovett-Barron M, Pfaff DW, Kow LM. Histaminergic responses by hypothalamic neurons that regulate lordosis and their modulation by estradiol. Proc Natl Acad Sci USA. 2010;107:12311–12316. doi: 10.1073/pnas.1006049107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox GB, Pan JB, Esbenshade TA, Bitner RS, Nikkel AL, Miller T, et al. Differential in vivo effects of H3 receptor ligands in a new mouse dipsogenia model. Pharmacol Biochem Behav. 2002;72:741–750. doi: 10.1016/s0091-3057(02)00745-1. [DOI] [PubMed] [Google Scholar]
- Fukagawa K, Sakata T, Shiraishi T, Yoshimatsu H, Fujimoto K, Ookuma K, et al. Neuronal histamine modulates feeding behavior through H1-receptor in rat hypothalamus. Am J Physiol. 1989;256:R605–R611. doi: 10.1152/ajpregu.1989.256.3.R605. [DOI] [PubMed] [Google Scholar]
- Garbarg M, Arrang JM, Rouleau A, Ligneau X, Tuong MD, Schwartz JC, et al. S-[2-(4-imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3 receptor agaonist. J Pharmacol Exp Ther. 1992;263:304–310. [PubMed] [Google Scholar]
- Gbahou F, Rouleau A, Morisset S, Parmentier R, Crochet S, Lin JS, et al. Protean agonism at histamine H3 receptors in vitro and in vivo. Proc Natl Acad Sci U S A. 2003;100:11086–11091. doi: 10.1073/pnas.1932276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Brune ME. Assessment of pharmacology and potential anti-obesity properties of H3 receptor antagonists/inverse agonists. Expert Opin Investig Drugs. 2005;14:223–241. doi: 10.1517/13543784.14.3.223. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Bennani YL, Bush EN, Esbenshade TA, Faghih R, Fox GB, et al. Antiobesity effects of A-331440, a novel non-imidazole histamine H3 receptor antagonist. Eur J Pharmacol. 2004;487:183–197. doi: 10.1016/j.ejphar.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Hancock AA, Diehl MS, Fey TA, Bush EN, Faghih R, Miller TR, et al. Antiobesity evaluation of histamine H3 receptor (H3R) antagonist analogs of A-331440 with improved safety and efficacy. Inflamm Res. 2005;54(Suppl. 1):S27–S29. doi: 10.1007/s00011-004-0412-z. [DOI] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29:14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MB, Zheng S, Duan C, Patel B, Vassileva G, Sondey C, et al. Antidiabetic properties of the histamine H3 receptor protean agonist proxyfan. Endocrinology. 2011;152:828–835. doi: 10.1210/en.2010-0757. [DOI] [PubMed] [Google Scholar]
- Ishizuka T, Hatano K, Murotani T, Yamatodani A. Comparison of the effect of an H(3)-inverse agonist on energy intake and hypothalamic histamine release in normal mice and leptin resistant mice with high fat diet-induced obesity. Behav Brain Res. 2008;188:250–254. doi: 10.1016/j.bbr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Itoh E, Fujimiya M, Inui A. Thioperamide, a histamine H3 receptor antagonist, suppresses NPY-but not dynorphin A-induced feeding in rats. Regul Pept. 1998;75-76:373–376. doi: 10.1016/s0167-0115(98)00090-1. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Oishi R, Saeki K. Feeding-induced increase in the extracellular concentration of histamine in rat hypothalamus as measured by in vivo microdialysis. Neurosci Lett. 1991;125:235–237. doi: 10.1016/0304-3940(91)90037-t. [DOI] [PubMed] [Google Scholar]
- Jang IS, Rhee JS, Watanabe T, Akaike N, Akaike N. Histaminergic modulation of GABAergic transmission in rat ventromedial hypothalamic neurones. J Physiol. 2001;534:791–803. doi: 10.1111/j.1469-7793.2001.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jethwa PH, Barrett P, Turnbull Y, Enright RA, Warner A, Murphy M, et al. The role of histamine 3 receptors in the control of food intake in a seasonal model of obesity: the Siberian hamster. Behav Pharmacol. 2009;20:155–165. doi: 10.1097/FBP.0b013e32832a8099. [DOI] [PubMed] [Google Scholar]
- Kent P, Plamondon H, Merali Z. Pharmaco-ontogeny of bombesin's suppression of food intake and its attenuation by histamine H3 receptor agonists. Brain Res Dev Brain Res. 1997;102:87–95. doi: 10.1016/s0165-3806(97)00084-9. [DOI] [PubMed] [Google Scholar]
- King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kow LM, Easton A, Pfaff DW. Acute estrogen potentiates excitatory responses of neurons in rat hypothalamic ventromedial nucleus. Brain Res. 2005;1043:124–131. doi: 10.1016/j.brainres.2005.02.068. [DOI] [PubMed] [Google Scholar]
- Lawrence CB, Ellacott KL, Luckman SM. PRL-releasing peptide reduces food intake and may mediate satiety signaling. Endocrinology. 2002;143:360–367. doi: 10.1210/endo.143.2.8609. [DOI] [PubMed] [Google Scholar]
- Lecklin A, Tuomisto L. The blockade of H1 receptors attenuates the suppression of feeding and diuresis induced by inhibition of histamine catabolism. Pharmacol Biochem Behav. 1998;59:753–758. doi: 10.1016/s0091-3057(97)00465-6. [DOI] [PubMed] [Google Scholar]
- Lecklin A, Etu-Seppala P, Stark H, Tuomisto L. Effects of intracerebroventricularly infused histamine and selective H1, H2 and H3 agonists on food and water intake and urine flow in Wistar rats. Brain Res. 1998;793:279–288. doi: 10.1016/s0006-8993(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Leurs R, Bakker RA, Timmerman H, de Esch IJ. The histamine H3 receptor: from gene cloning to H3 receptor drugs. Nat Rev Drug Discov. 2005;4:107–120. doi: 10.1038/nrd1631. [DOI] [PubMed] [Google Scholar]
- Ligneau X, Garbarg M, Vizuete ML, Diaz J, Purnad K, Stark H, et al. [125I]iodoproxyfan, a new antagonist to label and visualize cerebral histamine H3 receptors. J Pharmacol Exp Ther. 1994;271:452–459. [PubMed] [Google Scholar]
- Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, et al. Cloning and functional expression of the human histamine H3 receptor. Mol Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- Malmlof K, Zaragoza F, Golozoubova V, Refsgaard HH, Cremers T, Raun K, et al. Influence of a selective histamine H3 receptor antagonist on hypothalamic neural activity, food intake and body weight. Int J Obes (Lond) 2005;29:1402–1412. doi: 10.1038/sj.ijo.0803036. [DOI] [PubMed] [Google Scholar]
- Malmlof K, Golozoubova V, Peschke B, Wulff BS, Refsgaard HH, Johansen PB, et al. Increase of neuronal histamine in obese rats is associated with decreases in body weight and plasma triglycerides. Obesity (Silver Spring) 2006;14:2154–2162. doi: 10.1038/oby.2006.252. [DOI] [PubMed] [Google Scholar]
- Malmlof K, Hastrup S, Wulff BS, Hansen BC, Peschke B, Jeppesen CB, et al. Antagonistic targeting of the histamine H3 receptor decreases caloric intake in higher mammalian species. Biochem Pharmacol. 2007;73:1237–1242. doi: 10.1016/j.bcp.2007.01.034. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Banks K. Does the histaminergic system mediate bombesin/GRP-induced suppression of food intake? Am J Physiol. 1994;267:R1589–R1595. doi: 10.1152/ajpregu.1994.267.6.R1589. [DOI] [PubMed] [Google Scholar]
- Morisset S, Rouleau A, Ligneau X, Gbahou F, Tardivel-Lacombe J, Stark H, et al. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- Ookuma K, Sakata T, Fukagawa K, Yoshimatsu H, Kurokawa M, Machidori H, et al. Neuronal histamine in the hypothalamus suppresses food intake in rats. Brain Res. 1993;628:235–242. doi: 10.1016/0006-8993(93)90960-u. [DOI] [PubMed] [Google Scholar]
- Orthen-Gambill N, Salomon M. FMH-induced decrease in central histamine levels produces increased feeding and body weight in rats. Physiol Behav. 1992;51:891–893. doi: 10.1016/0031-9384(92)90132-l. [DOI] [PubMed] [Google Scholar]
- Passani MB, Blandina P, Torrealba F. The histamine H3 receptor and eating behavior. J Pharmacol Exp Ther. 2011;336:24–29. doi: 10.1124/jpet.110.171306. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1986. [Google Scholar]
- Pillot C, Heron A, Cochois V, Tardivel-Lacombe J, Ligneau X, Schwartz JC, et al. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114:173–193. doi: 10.1016/s0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- Poole SL, Lewis DI, Deuchars SA. Histamine depolarizes neurons in the dorsal vagal complex. Neurosci Lett. 2008;432:19–24. doi: 10.1016/j.neulet.2007.11.055. [DOI] [PubMed] [Google Scholar]
- Sakata T, Ookuma K, Fukagawa K, Fujimoto K, Yoshimatsu H, Shiraishi T, et al. Blockade of the histamine H1-receptor in the rat ventromedial hypothalamus and feeding elicitation. Brain Res. 1988;441:403–407. doi: 10.1016/0006-8993(88)91423-0. [DOI] [PubMed] [Google Scholar]
- Sakata T, Fukagawa K, Ookuma K, Fujimoto K, Yoshimatsu H, Yamatodani A, et al. Hypothalamic neuronal histamine modulates ad libitum feeding by rats. Brain Res. 1990;537:303–306. doi: 10.1016/0006-8993(90)90373-j. [DOI] [PubMed] [Google Scholar]
- Scott V, Kimura N, Stark JA, Luckman SM. Intravenous peptide YY3-36 and Y2 receptor antagonism in the rat: effects on feeding behaviour. J Neuroendocrinol. 2005;17:452–457. doi: 10.1111/j.1365-2826.2005.01330.x. [DOI] [PubMed] [Google Scholar]
- Sheiner JB, Morris P, Anderson GH. Food intake suppression by histidine. Pharmacol Biochem Behav. 1985;23:721–726. doi: 10.1016/0091-3057(85)90061-9. [DOI] [PubMed] [Google Scholar]
- Sindelar DK, Shepperd ML, Pickard RT, Alexander-Chacko J, Dill MJ, Cramer JW, et al. Central H3R activation by thioperamide does not affect energy balance. Pharmacol Biochem Behav. 2004;78:275–283. doi: 10.1016/j.pbb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Suwa H, Ishikawa T, Kotani H. Targeted disruption of H3 receptors results in changes in brain histamine tone leading to an obese phenotype. J Clin Invest. 2002;110:1791–1799. doi: 10.1172/JCI200215784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye C, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger TJ, Calderon GA, Bradley LC, Sena-Esteves M, Rios M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff BS, Hastrup S, Rimvall K. Characteristics of recombinantly expressed rat and human histamine H3 receptors. Eur J Pharmacol. 2002;453:33–41. doi: 10.1016/s0014-2999(02)02382-8. [DOI] [PubMed] [Google Scholar]
- Yoshimoto R, Miyamoto Y, Shimamura K, Ishihara A, Takahashi K, Kotani H, et al. Therapeutic potential of histamine H3 receptor agonist for the treatment of obesity and diabetes mellitus. Proc Natl Acad Sci USA. 2006;103:13866–13871. doi: 10.1073/pnas.0506104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto R, Kanatani A, Tokita S. Distinctive role of central histamine H3 receptor in various orexigenic pathways. Eur J Pharmacol. 2008;579:229–232. doi: 10.1016/j.ejphar.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee AW, Devidze N, Zhang Q, Kow LM, Pfaff DW. Histamine-induced excitatory responses in mouse ventromedial hypothalamic neurons: ionic mechanisms and estrogenic regulation. J Neurophysiol. 2007;98:3143–3152. doi: 10.1152/jn.00337.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


