Abstract
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of growth factors that bind to and activate the EGF receptor. HB-EGF is synthesized as a membrane-anchored protein (proHB-EGF), and then proteolytically cleaved, resulting in the mitogenically active soluble form. HB-EGF plays pivotal roles in pathophysiological processes such as development and cell proliferation. In this study, we developed an immuno-PCR system for the determination of soluble HB-EGF concentrations in human serum. Utilizing a monoclonal antibody with neutralizing activity against HB-EGF as a capture antibody resulted in increasing selectivity for an active form of HB-EGF in serum, and immuno-PCR system improved its sensitivity compared to the currently available methods. As a result of measurement of HB-EGF in 20 ovarian cancer patients and 20 healthy volunteers, ovarian cancer patients showed significantly higher concentrations than healthy volunteers (P< 0.05), which indicates that soluble HB-EGF detected by newly developed immuno-PCR system can be useful serological biomarkers such as a diagnostic biomarker for ovarian cancer, and a predictive and pharmacodynamic biomarker for anti-HB-EGF targeted therapies under development.
Keywords: HB-EGF, ovarian cancer, serological biomarker, immuno-PCR
Introduction
Epidermal growth factor (EGF) receptors (ErbB) and EGF family members represent promising targets for anticancer therapy. Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family and is an important therapeutic target in some types of human cancers. HB-EGF binds to and activates both HER1 and HER4 [1-3], and plays a pivotal role in many physiologic and pathologic processes via transduction of extracellular signals [4-6]. HB-EGF has been reported to be involved in a number of pathological processes such as cardiac hypertrophy [7] and tumorigenesis in ovarian cancer [8,9].
Like other members of EGF family, HB-EGF is synthesized as a membrane-anchored protein (proHB-EGF), composed of a signal peptide, propeptide, heparin-binding, EGF-like, juxtamembrane, transmembrane, and cytoplasmic domains [1]. ProHB-EGF is cleaved at its juxtamembrane domain by metalloproteases in a process called ectodomain shedding [10]. Ectodomain shedding of proHB-EGF yields a soluble form of HB-EGF, which is a potent mitogen and chemo-attractant for cells expressing its cognate ErbB receptors [4].
HB-EGF, especially its soluble form, was reported to be essential for tumor growth of ovarian cancer cells in nude mice [8]. In addition, among members of the EGF family of growth factors, HB-EGF gene expression in cancerous tissues and HB-EGF protein levels were shown to be significantly higher in patients’ ascites [8,11]. Furthermore, tumor formation by human ovarian cancer cell lines was enhanced by exogenous expression of proHB-EGF and completely blocked by proHB-EGF gene RNA interference or by CRM197, a specific HB-EGF inhibitor [8]. It has also been shown that HB-EGF expression is significantly associated with the clinical outcome in ovarian cancer [12]. Based on these evidences, HB-EGF is now considered to be an attractive therapeutic target in human. In this study, we developed a novel method with high sensitivity and specificity for the determination of soluble HB-EGF concentrations in human serum.
In order to develop a sandwich ligand binding assay method, we chose a combination of the two antibodies; a mouse monoclonal anti-HB-EGF antibody as a capture antibody and a goat polyclonal anti-HB-EGF antibody as a detection antibody. First, we developed a sandwich ELISA with horseradish peroxidase detection system using the two antibodies described above. However, no endogenous HB-EGF was detected in sera of ovarian cancer patients and healthy volunteers due to the low sensitivity. We then sensitized the method by introducing immuno-PCR, resulting in the change of lower limit of quantification (LLOQ) from 100 to 5 pg/mL. Immuno-PCR is a method which combines the specificity of antibodies with the sensitivity of PCR. There are some previous reports on the method development of immuno-PCR [13,14].
Using our method, we quantified soluble HB-EGF levels in healthy volunteers and ovarian cancer patients. As a result, we demonstrated that ovarian cancer patients showed significantly higher level of HB-EGF than healthy volunteers.
This is the report suggesting that soluble HB-EGF detected by newly developed immuno-PCR system can be a clear-cut serological biomarkers for ovarian cancer.
Materials and methods
Materials
A goat polyclonal anti-HB-EGF antibody and recombinant human HB-EGF were purchased from R&D systems (Minneapolis, MN, USA). A mouse monoclonal anti-HB-EGF antibody, KM3566 (Patent Application Publication No. US 2011/0052595 A1) was obtained from Kyowa Hakko Kirin (Tokyo, Japan). EGF was purchased from Invitrogen (Carlsbad, CA, USA). Hepatocyte growth factor (HGF) and transforming growth factor-alpha (TGF-α) were purchased from R&D systems.
Matrix
Healthy volunteers’ sera were purchased from Uniglobe Research Corporation (Reseda, CA, USA). Ovarian cancer patients’ sera were purchased from Bioreclamation (Hicksville, NY, USA).
Determination of soluble HB-EGF concentrations in human serum by immuno-PCR system
A DNA conjugate was prepared for a detection antibody. An 80-base long single stranded DNA with amino-modification at the 5’-end was linked covalently to a goat polyclonal anti-HB-EGF antibody (R&D systems) using Antibody-Oligonucleotide All-in-One™ Conjugation Kit (Solulink, San Diego, CA, USA) according to the manufacturer’s protocol. The DNA sequence was 5’-GGG GAA TTC GTT AGC CGG GCT GCA CTC AAT GTA CAC CGA CAT GTG GAG TGA AGA GTA TCA GTG TGC ATG GCT GGA TAT GT-3’ (Sigma-Aldrich Japan, Tokyo, Japan).
A capture antibody (KM3566) was added to the wells of the microtiter plate, Nunc Maxisorp (Thermo Fisher Scientific, Yokohama, Japan), and incubated for 1 hour at room temperature. The wells were washed three times with wash buffer, and the surface was blocked with PBS containing 1% casein. After washing three times, calibration standards and analytical samples were added to each well of the plate and incubated for 1 hour at room temperature. The plate was then washed three times, followed by addition of detection antibody (DNA-conjugated polyclonal anti-HB-EGF antibody). After incubation for 1 hour at room temperature, the plate was washed three times. Thereafter, dithiothreitol solution was added to each well and incubated for 30 min at 37°C, and then, SYBR® Premix Ex Taq (Takara, Japan) with primers below was added to each well. Forward primer: 5’-GGG AAT TCG TTA GCC GGG-3’ (Sigma-Aldrich Japan). Reverse primer: 5’-ACA TAT CCA GCC ATG CAC AC-3’ (Sigma-Aldrich Japan).
Finally, a part of the mixture in each well was transferred to each well of the PCR plate, Micro-Amp optical 96-well reaction plate (Applied Biosystems, Tokyo, Japan), followed by real-time PCR under the following condition using Realtime PCR cycler, ABI Prism 7000® (Applied Biosystems). 95°C for 30 s (1 cycle). 95°C for 5 s, 58°C for 30 s, 72°C for 30 s (40 cycles). The LLOQ was set to be 5 pg/mL. A standard curve was created using SoftMax® Pro (Nihon Molecular Devices, Tokyo, Japan) capable of generating a four-parameter logistic curve-fit.
Other methods
Statistical analysis was conducted using Microsoft Excel 2003.
Results
Method validation of immuno-PCR system for the determination of soluble HB-EGF concentrations in human serum
A typical standard curve of this assay for determination of HB-EGF concentrations is shown in Figure 1. The intra- and inter- assay accuracy (RE; %) and coefficient of variation (CV; %) were calculated by measuring three different concentrations (n=5 at each concentration) with recombinant human HB-EGF protein (Table 1). The RE and CV values of the intra-assay ranged from -17.9% to -10.0% and from 7.5% to 25.7%, respectively. The RE and CV values of the three independent inter-assay ranged from -10.8% to -8.6% and from 9.9% to 17.4%, respectively. Matrix interference was confirmed by adding recombinant HB-EGF to 6 individual human sera. As a result, the recovery ranged from 95% to 113%, suggesting no matrix interference. Moreover, addition of recombinant human TGF-α, EGF, and HGF did not affect a measurement in the range of 5-200 pg/mL (Figure 2) and at 1 μg/mL (data not shown). These results indicate that soluble HB-EGF can be measured by an immuno-PCR method not only with acceptable accuracy and precision but also with high sensitivity and specificity in the presence of 50% human serum. The lower limit of quantification was 5 pg/mL.
Figure 1.
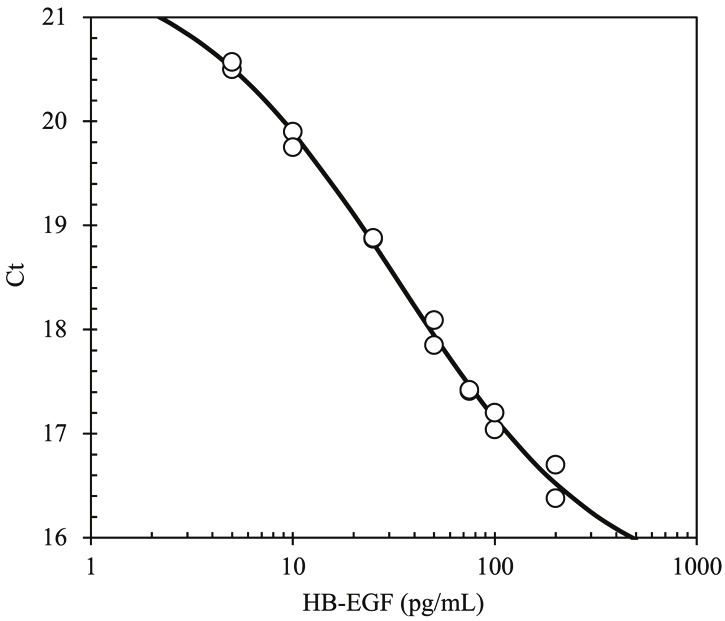
A typical standard curve for the determination of HB-EGF concentrations by immuno-PCR method. Concentrations of calibration standards for HB-EGF are 5, 10, 25, 50, 75, 100, and 200 pg/mL. The standard curve was generated by a four-parameter logistic curve-fit. (Ct; threshold cycle).
Table 1.
The intra- and inter- assay accuracy (RE; %) and coefficient of variation (CV; %) calculated by measuring three different concentrations with recombinant human HB-EGF protein
| Intra-assay accuracy and precision | |||
|
| |||
| Added conc. (pg/mL) | 100 | 25 | 5 |
| Mean (n=5) | 82.1 | 22.5 | 4.28 |
| SD | 6.18 | 2.64 | 1.10 |
| aRE (%) | -17.9 | -10.0 | -14.4 |
| bCV (%) | 7.5 | 11.7 | 25.7 |
|
| |||
| Inter-assay accuracy and precision | |||
|
| |||
| Added conc. (pg/mL) | 100 | 25 | 5 |
| Mean (n=5) | 91.4 | 22.3 | 4.48 |
| SD | 10.1 | 2.20 | 0.779 |
| aRE (%) | -806 | -10.8 | -10.4 |
| bCV (%) | 11.1 | 9.9 | 17.4 |
RE (%) = (Observed values-Nominal values) / Nominal values x 100;
CV (%) = Standard deviation / Mean observed values x 100.
Figure 2.
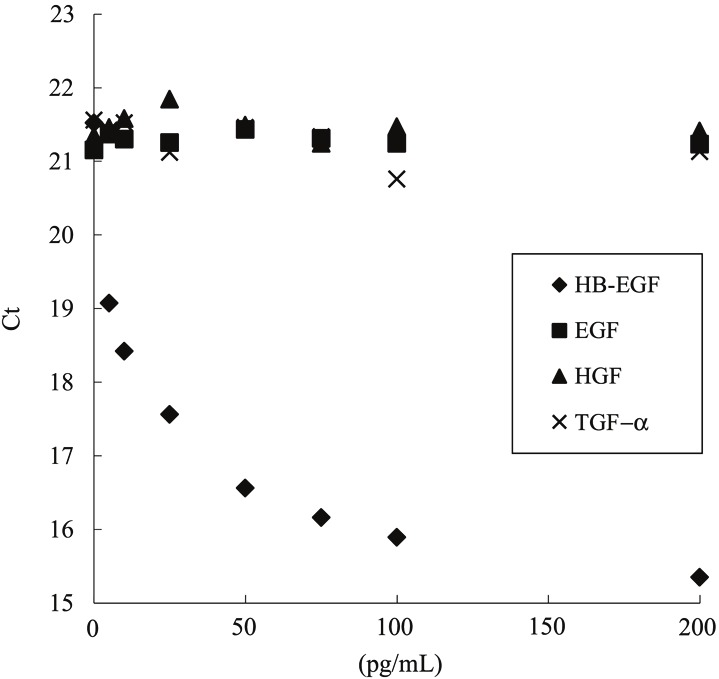
Confirmation of specificity by addition of recombinant human HB-EGF, TGF-α, EGF, and HGF. The method showed no cross-reactivity with other EGF family proteins such as EGF and TGF-α, and had no cross-reactivity with HGF which has an affinity for heparin. (Ct; threshold cycle).
Stability of soluble HB-EGF in human serum
Serum samples of ovarian cancer patients subjected to long-term storage up to 3 months at - 80°C, short-term storage up to 24 hours at room temperature, and 3 cycles of freeze-andthaw were compared to their fresh samples. Each remaining (%) was calculated by dividing observed concentrations of tested samples by their initial values. The averages of the remaining percentages in five samples of 1-, 2-, and 3-month storage at -80°C were 96.8%, 91.3%, and 98.7%, respectively. The averages of the remaining percentages in three samples of 24-hour storage at room temperature and 3- cycle freeze-and-thaw were 91.3% and 109.2%, respectively. These results suggested that endogenous soluble HB-EGF is fairly stable in human serum.
Determination of soluble HB-EGF concentrations in human serum by immuno-PCR method
Levels of soluble HB-EGF in human serum were quantified by the immuno-PCR method. Serum samples whose soluble HB-EGF levels were below the LLOQ were regarded as LLOQ, 5 pg/mL. Figure 3 shows a comparison between healthy volunteers (n=20) and ovarian cancer patients (n=20). The mean soluble HB-EGF levels of the 20 healthy volunteers and 20 ovarian cancer patients were 5.36 pg/mL and 28.6 pg/mL, respectively. The individual HB-EGF levels of patients’ sera examined are shown in Table 2 with the information of clinical characteristics provided by the vendor of the sera. The values were analyzed by Welch’s t test. The result of the t test was P<0.05, suggesting that there was a statistically significant difference between the values of healthy volunteers and ovarian cancer patients.
Figure 3.
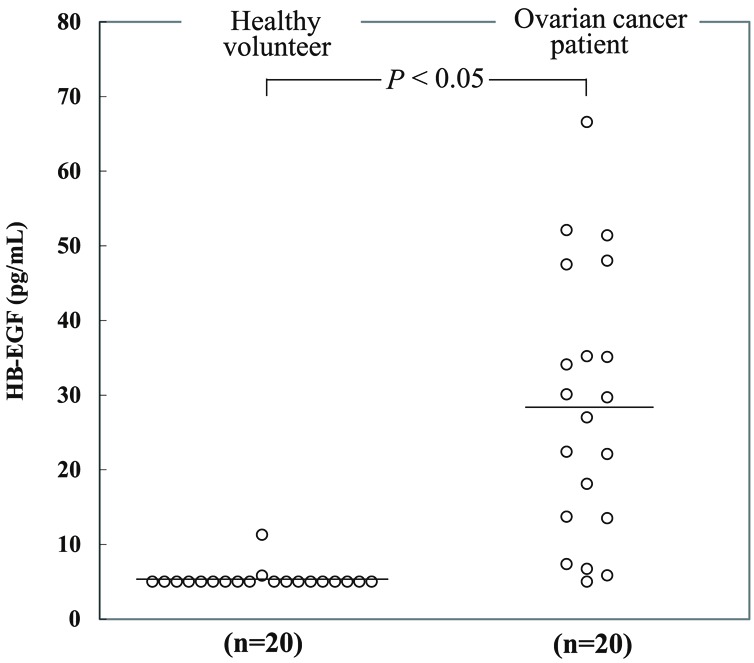
Comparison of serum HB-EGF concentrations determined by immuno-PCR method between healthy volunteers (n=20) and ovarian cancer patients (n=20). The lower limit of quantification was 5 pg/mL, and serum HB-EGF levels below the limit of quantification were regarded as 5 pg/mL. Open circles represent the individual HB-EGF levels. Bars indicate mean. The result of Welch’s t test was P<0.05, suggesting that there was a statistically significant difference between the values of healthy volunteers and ovarian cancer patients.
Table 2.
Clinical characteristics of ovarian cancer patients examined and HB-EGF concentrations determined by immuno-PCR
| Lot No. | Age | Medication | Stage and condition | HB-EGF (pg/mL) |
|---|---|---|---|---|
| BRH469170 | 76 | Neulasta, Lisinopril, Calcium, Fish oil, Crestor, Valtrex | Flare-up: IV recurrence | 7.35 |
| BRH469171 | 60 | Lisinopril, ASA, Atenolol, Nifedipine, Miralax, Vitamins, Clonidine | Stable: IIIC, T2cNIMX | 30.1 |
| BRH469172 | 57 | Valtrex, Premarin, Coumadin, Ambien, Restasis, Gelclair Gel | IV, Stable | 66.6 |
| BRH469173 | 61 | levothyroxine, provastatin | Remission | 35.1 |
| BRH469174 | 75 | Synthroid, Lisinopril, Omeprazole, Celexa, ASA, Multivitamin | Stable | 52.1 |
| BRH469175 | 54 | Metformin, Travatan, Crestor, Alphagen, Insulin, Victosa, Micardis, Neulasta | Remission | 47.5 |
| BRH469176 | 75 | Enalapril, Centrum Silver, Vitamin B-12, B-6, Calcium Citrate | IV, Stable | 51.4 |
| BRH410632 | 42 | Doxil | IV | 22.1 |
| BRH410633 | 67 | Taxol, Carboplatin | III | 18.1 |
| BRH410635 | 84 | Taxol, Carboplatin | II | 22.4 |
| BRH501381 | 74 | Carboplatin, Synthroid, Taxol, Metoprolol, Heparin Lock, Simvastatin, Metformin | III | 34.2 |
| BRH501382 | 68 | Xanax, Coumadin, Bystolic, Carboplatin, Doxil, Avastin | IV | 14.0 |
| BRH501383 | 63 | Carvedilol, Tamoxifen | III | 14.2 |
| BRH501384 | 53 | Taxol | IIIB | 30.0 |
| BRH501385 | 72 | Glipizide, Metoprolol Succinate, Haldol | III | 5.79 |
| BRH501386 | 55 | Synthroid, Taxol, Carboplatin | IA | <LLOQ |
| BRH501387 | 57 | Coumadin, Endocet | IIIC | 6.76 |
| BRH501388 | 93 | Atenolol, Doxazosin, Lasix, Synthroid, Omeprazole, ASA, Vitamin C, Vitamin D, Oxybitymine, Magnesium, Potassium, Simvastatin, Aricept, Hycamtin | III | 27.4 |
| BRH501389 | 61 | Toprol, Digoxin, Femara, Ameprazole | II | 47.4 |
| BRH501390 | 66 | Crestor, Omega 3, Calcium, Vitamins, ASA, Glucosamine | III | 35.3 |
Discussion
In this study, we successfully developed a highly sensitive immuno-PCR method for the determination of soluble HB-EGF concentrations in human serum with acceptable accuracy and precision (Table 1). The method showed no cross-reactivity with other EGF family proteins such as EGF and TGF-α, and had no cross-reactivity with HGF which has an affinity for heparin (Figure 2).
With our newly developed method, we measured soluble HB-EGF in human serum and demonstrated a statistically significant difference in soluble HB-EGF concentrations between sera of healthy volunteers and ovarian cancer patients (Figure 3).
There are some academic papers reporting about human serum or plasma concentrations of HB-EGF. Yamada et al. developed a sandwich ELISA combined with enrichment process by using heparin and demonstrated that the mean plasma HB-EGF concentration of the 95 healthy volunteers was 15.9 pg/mL [15]. Matsumoto et al. demonstrated that mean plasma HB-EGF levels in the coronary artery disease (CAD) patients and non-CAD patients were 164.3 and 62.6 pg/mL, respectively, with the same method as above [16]. On the other hand, a competitive ELISA developed by Sanchez-vizcaino et al. determined serum HB-EGF in the range of 0.03-3 nM and the mean serum HB-EGF concentrations in women and men were 0.26 nM and 0.28 nM (approx. 3 ng/mL), respectively [17]. According to a report from Keay et al., the mean concentrations of soluble HB-EGF in sera from interstitial cystitis cases and normal controls were 2.3 and 9.1 ng/mL, respectively [18]. Another report from the same author’s group showed that the mean urine concentration of soluble HB-EGF was strikingly lower in interstitial cystitis patient specimens (1.53 ng/mL) as compared to asymptomatic controls (6.33 ng/mL) or patients with bacterial cystitis (5.15 ng/mL) [19]. Their method is not a sandwich ELISA but a sample (e.g. serum, cell lysate, or urine)-coated ELISA which seems to prone to produce non-specificity. The differences in the range of HB-EGF levels in serum or plasma between previous reports seem to be due to the difference in measurement principle. Our method utilizes KM3566 as a capture antibody. KM3566 neutralizes HB-EGF activity in a way KM3566 inhibits the binding of HB-EGF to its receptors [20]. Therefore, only an active form of soluble HB-EGF, which has a potency to bind to its receptors, should be captured by KM3566, and then, detected by the polyclonal anti-HB-EGF antibody. In our recent report, we demonstrated that soluble HB-EGF levels in sera of ovarian cancer patients were higher than those in controls using the commercially available kit with some modifications of protocol [21]. Compared to the results with the kit in the previous study, soluble HB-EGF levels determined by the newly developed immuno-PCR in this study were relatively lower but the difference between healthy volunteers and ovarian cancer patients was clearer. This can be explained that our newly developed method detects only an active form of soluble HB-EGF which may lead to pathogenesis of diseases while the previous method utilizing a combination of polyclonal antibodies theoretically detects any forms of HB-EGF molecule regardless of their reactivity against receptors. This interpretation possibly suggests that soluble HB-EGF levels obtained by our newly developed method should be more meaningful in terms of disease prognosis.
There have been some reports regarding biological activities of HB-EGF. In cell culture, soluble HB-EGF is a potent mitogen (ED50 of 0.01-10 ng/mL) for a number of cell types ranging from epithelial cells, hepatocytes, and keratinocytes to breast and ovarian carcinoma cells [4]. Considering the ED50 values reported, the concentration range of soluble HB-EGF in the sera of ovarian cancer patients detected by our newly developed method should be meaningful.
Although correlation between serum HB-EGF levels and clinical characteristics (e.g. age, history of medications, disease stage, and current condition) remains to be explored in larger cohorts, serum HB-EGF was detected in the patients of the early stage (e.g. stage II) (Table 2). Therefore, serum HB-EGF detected by the newly developed method can be an early diagnostic marker for ovarian cancer.
HB-EGF is also expressed in a variety of human carcinomas such as pancreatic, esophageal, colon, gastric, and bladder cancers [9], and targeting it therapeutically is under clinical evaluation [22]. Moreover, HB-EGF mRNA is reported to be increased in human pancreatic cancer in comparison to normal pancreas, suggesting that HB-EGF may be an important contributor to pancreatic cancer cell growth [23]. In addition, it is also highly expressed in hepatoma tissues but not in normal liver, indicating that HB-EGF is associated with the progression of hepatocarcinogenesis [24]. Exploring soluble HB-EGF concentration in these populations should be of our next interest.
In summary, we demonstrated a statistically significant difference in soluble HB-EGF concentrations between sera of healthy volunteers and ovarian cancer patients. Therefore, it is possible that soluble HB-EGF detected by newly developed immuno-PCR system can be serological biomarkers such as a diagnostic biomarker for ovarian cancer, and a predictive and pharmacodynamic biomarker for anti-HB-EGF targeted therapies under development.
Acknowledgements
We thank Drs. Furuya A, Ishida H, Ohki Y, Kanda H, Ushiki J, and Kaito H for excellent support and helpful suggestion.
References
- 1.Higashiyama S, Lau K, Besner GE, Abraham JA, Klagsbrun M. Structure of heparin-binding EGF-like growth factor. Multiple forms, primary structure, and glycosylation of the mature protein. J Biol Chem. 1992;267:6205–6212. [PubMed] [Google Scholar]
- 2.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–939. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 3.Elenius K, Paul S, Allison G, Sun J, Klagsbrun M. Activation of HER4 by heparin-binding EGF-like growth factor stimulates chemotaxis but not proliferation. EMBO J. 1997;16:1268–1278. doi: 10.1093/emboj/16.6.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raab G, Klagsbrun M. Heparin-binding EGF-like growth factor. Biochim Biophys Acta. 1997;1333:F179–199. doi: 10.1016/s0304-419x(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 5.Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 6.Higashiyama S, Nanba D. ADAM-mediated ectodomain shedding of HB-EGF in receptor crosstalk. Biochim Biophys Acta. 2005;1751:110–117. doi: 10.1016/j.bbapap.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Asakura M, Kitakaze M, Takashima S, Liao Y, Ishikura F, Yoshinaka T, Ohmoto H, Node K, Yoshino K, Ishiguro H, Asanuma H, Sanada S, Matsumura Y, Takeda H, Beppu S, Tada M, Hori M, Higashiyama S. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto S, Hirata M, Yamazaki A, Kageyama T, Hasuwa H, Mizushima H, Tanaka Y, Yagi H, Sonoda K, Kai M, Kanoh H, Nakano H, Mekada E. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004;64:5720–5727. doi: 10.1158/0008-5472.CAN-04-0811. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto S, Yagi H, Yotsumoto F, Kawarabayashi T, Mekada E. Heparin-binding epidermal growth factor-like growth factor as a novel targeting molecule for cancer therapy. Cancer Sci. 2006;97:341–347. doi: 10.1111/j.1349-7006.2006.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goishi K, Higashiyama S, Klagsbrun M, Nakano N, Umata T, Ishikawa M, Mekada E, Taniguchi N. Phorbol ester induces the rapid processing of cell surface heparin-binding EGF-like growth factor: conversion from juxtacrine to paracrine growth factor activity. Mol Biol Cell. 1995;6:967–980. doi: 10.1091/mbc.6.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yagi H, Miyamoto S, Tanaka Y, Sonoda K, Kobayashi H, Kishikawa T, Iwamoto R, Mekada E, Nakano H. Clinical significance of heparin-binding epidermal growth factor-like growth factor in peritoneal fluid of ovarian cancer. Br J Cancer. 2005;92:1737–1745. doi: 10.1038/sj.bjc.6602536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka Y, Miyamoto S, Suzuki SO, Oki E, Yagi H, Sonoda K, Yamazaki A, Mizushima H, Maehara Y, Mekada E, Nakano H. Clinical significance of heparin-binding epidermal growth factor-like growth factor and a disintegrin and metalloprotease 17 expression in human ovarian cancer. Clin Cancer Res. 2005;11:4783–4792. doi: 10.1158/1078-0432.CCR-04-1426. [DOI] [PubMed] [Google Scholar]
- 13.Sims PW, Vasser M, Wong WL, Williams PM, Meng YG. Immunopolymerase chain reaction using real-time polymerase chain reaction for detection. Anal Biochem. 2000;281:230–232. doi: 10.1006/abio.2000.4578. [DOI] [PubMed] [Google Scholar]
- 14.Lind K, Kubista M. Development and evaluation of three real-time immuno-PCR assemblages for quantification of PSA. J Immunol Methods. 2005;304:107–116. doi: 10.1016/j.jim.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Yamada A, Kawata S, Tamura S, Kiso S, Higashiyama S, Umeshita K, Sakon M, Taniguchi N, Monden M, Matsuzawa Y. Plasma heparin-binding EGF-like growth factor levels in patients after partial hepatectomy as determined with an enzyme-linked immunosorbent assay. Biochem Biophys Res Commun. 1998;246:783–787. doi: 10.1006/bbrc.1998.8703. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Kishida K, Shimomura I, Maeda N, Nagaretani H, Matsuda M, Nishizawa H, Kihara S, Funahashi T, Matsuzawa Y. Increased plasma HB-EGF associated with obesity and coronary artery disease. Biochem Biophys Res Commun. 2002;292:781–786. doi: 10.1006/bbrc.2002.6720. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Vizcaino E, Vehi C, Camprecios G, Morcillo C, Soley M, Ramirez I. Heparin-binding EGF-like growth factor in human serum. Association with high blood cholesterol and heart hypertrophy. Growth Factors. 2010;28:98–103. doi: 10.3109/08977190903443030. [DOI] [PubMed] [Google Scholar]
- 18.Keay S, Kleinberg M, Zhang CO, Hise MK, Warren JW. Bladder epithelial cells from patients with interstitial cystitis produce an inhibitor of heparin-binding epidermal growth factor-like growth factor production. J Urol. 2000;164:2112–2118. [PubMed] [Google Scholar]
- 19.Keay S, Zhang CO, Kagen DI, Hise MK, Jacobs SC, Hebel JR, Gordon D, Whitmore K, Bodison S, Warren JW. Concentrations of specific epithelial growth factors in the urine of interstitial cystitis patients and controls. J Urol. 1997;158:1983–1988. doi: 10.1016/s0022-5347(01)64198-3. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S, Iwamoto R, Furuya A, Takahashi K, Sasaki Y, Ando H, Yotsumoto F, Yoneda T, Hamaoka M, Yagi H, Murakami T, Hori S, Shitara K, Mekada E. A novel anti-human HB-EGF monoclonal antibody with multiple antitumor mechanisms against ovarian cancer cells. Clin Cancer Res. 2011;17:6733–6741. doi: 10.1158/1078-0432.CCR-11-1029. [DOI] [PubMed] [Google Scholar]
- 21.Hikita S, Yotsumoto F, Fumaki T, Horiuchi S, Sanui A, Miyata K, Nam SO, Tsujioka H, Ueda T, Shirota K, Yoshizato T, Maeda K, Ishikawa T, Okuno Y, Kuroki M, Mekada E, Miyamoto S. Assessment of HB-EGF levels in peritoneal fluid and serum of ovarian cancer patients using ELISA. Anticancer Res. 2011;31:2553–2559. [PubMed] [Google Scholar]
- 22.Yagi H, Yotsumoto F, Sonoda K, Kuroki M, Mekada E, Miyamoto S. Synergistic anti-tumor effect of paclitaxel with CRM197, an inhibitor of HB-EGF, in ovarian cancer. Int J Cancer. 2009;124:1429–1439. doi: 10.1002/ijc.24031. [DOI] [PubMed] [Google Scholar]
- 23.Kobrin MS, Funatomi H, Friess H, Buchler MW, Stathis P, Korc M. Induction and expression of heparin-binding EGF-like growth factor in human pancreatic cancer. Biochem Biophys Res Commun. 1994;202:1705–1709. doi: 10.1006/bbrc.1994.2131. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi E, Higashiyama S, Nakagawa T, Suzuki K, Horimoto M, Hayashi N, Fusamoto H, Kamada T, Taniguchi N. High expression of heparin-binding EGF-like growth factor in rat hepatocarcinogenesis. Int J Cancer. 1996;68:215–218. doi: 10.1002/(SICI)1097-0215(19961009)68:2<215::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]


