Abstract
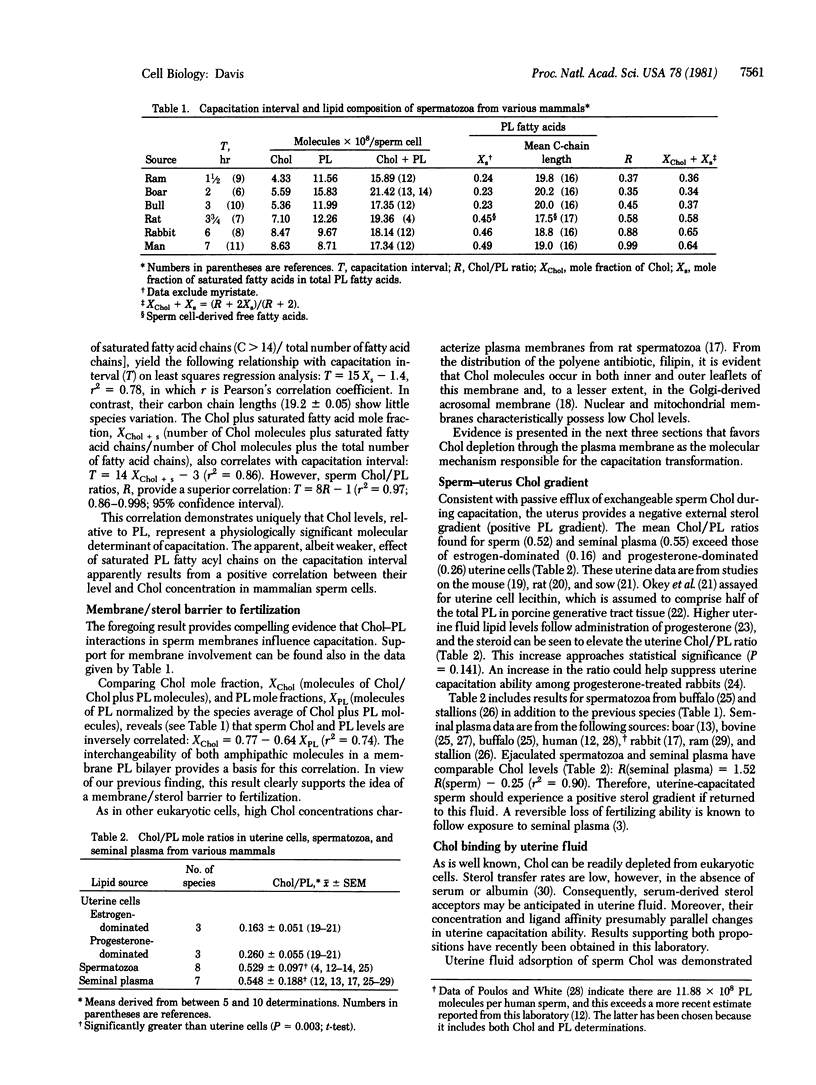
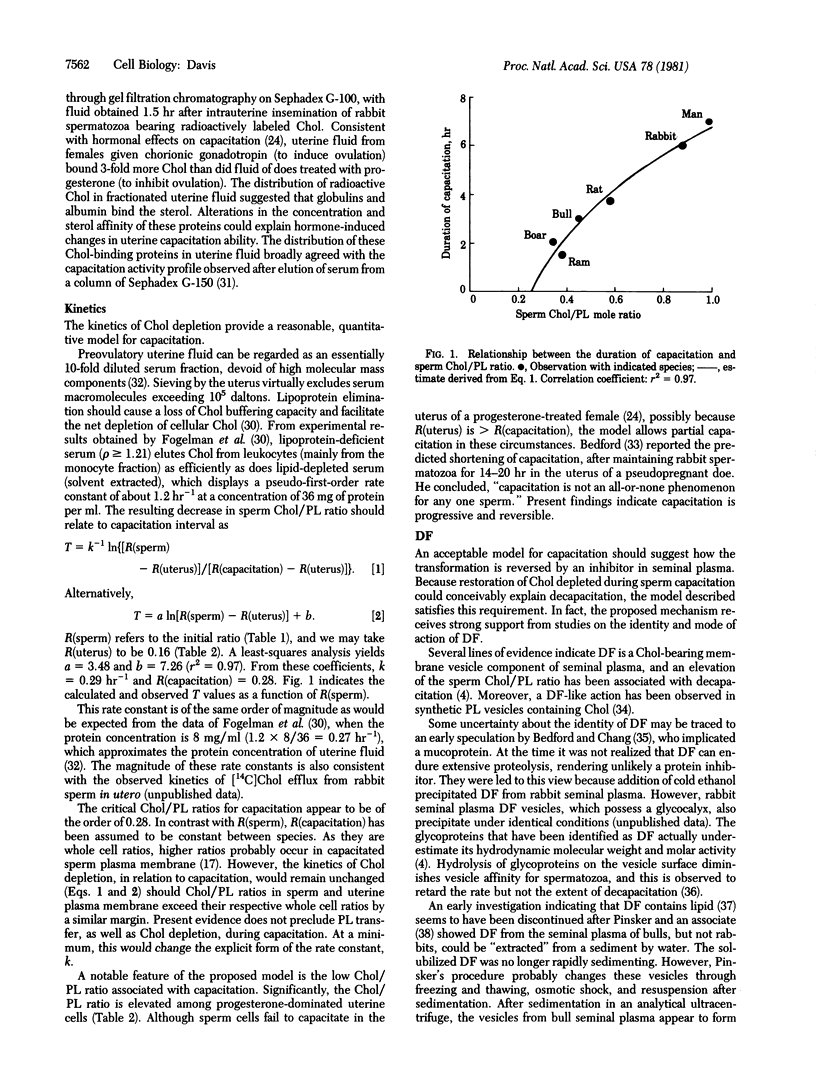
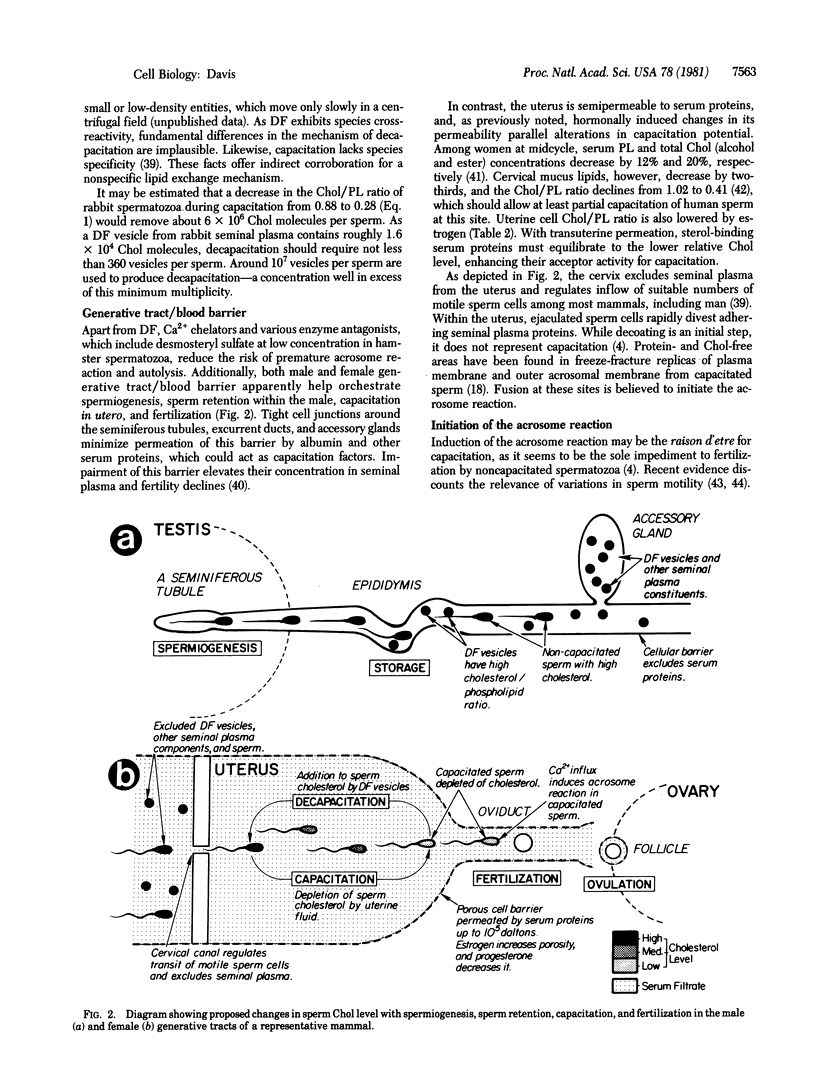
A survey of species differences in the duration of capacitation, T, has revealed that they closely correlate with sperm cholesterol/phospholipid mole ratios, R : T = 8R - 1 (r2 = 0.97, in which r is Pearson's correlation coefficient). Because uterine cells displayed low relative cholesterol concentrations, spermatozoa evidently experience a negative external cholesterol gradient (positive phospholipid gradient) during capacitation. A decrease in sperm R-value is suggested, therefore, to accompany capacitation. The idea received strong support from a kinetic analysis of capacitation intervals, based on the rate of cholesterol efflux from sperm cells in utero. Lipid-binding serum proteins in uterine fluid are attributed with removing a sterol barrier to the Ca2+-facilitated membrane fusion that initiates the acrosome reaction. Tight cell junctions prevent permeation of the male generative tract by these proteins (capacitation factors). Furthermore, seminal plasma contains a decapacitation factor, identified as a membrane vesicle (cholesterol donor) component of this fluid, that reverses capacitation. Initiation of the sperm acrosome reaction among mammals could be the first fusion process found to be physiologically modulated through the membrane bilayer cholesterol level.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R., BRADEN A. W. Time relations and their significance in the ovulation and penetration of eggs in rats and rabbits. Aust J Biol Sci. 1954 May;7(2):179–194. doi: 10.1071/bi9540179. [DOI] [PubMed] [Google Scholar]
- Austin C. R. Principles of fertilization. Proc R Soc Med. 1974 Sep;67(9):925–927. [PMC free article] [PubMed] [Google Scholar]
- BEDFORD J. M., CHANG M. C. Removal of decapacitation factor from seminal plasma by high-speed centrifugation. Am J Physiol. 1962 Jan;202:179–181. doi: 10.1152/ajplegacy.1962.202.1.179. [DOI] [PubMed] [Google Scholar]
- Bavister B. D., Edwards R. G., Steptoe P. C. Identification of the midpiece and tail of the spermatozoon during fertilization of human eggs in vitro. J Reprod Fertil. 1969 Oct;20(1):159–160. doi: 10.1530/jrf.0.0200159. [DOI] [PubMed] [Google Scholar]
- Bavister B. D., Morton D. B. Separation of human serum components capable of inducing the acrosome reaction in hamster spermatozoa. J Reprod Fertil. 1974 Oct;40(2):495–498. doi: 10.1530/jrf.0.0400495. [DOI] [PubMed] [Google Scholar]
- CHANG M. C. A detrimental effect of seminal plasma on the fertilizing capacity of sperm. Nature. 1957 Feb 2;179(4553):258–259. doi: 10.1038/179258a0. [DOI] [PubMed] [Google Scholar]
- CHANG M. C. Capacitation of rabbit spermatozoa in the uterus with special references to the reproductive phases of the female. Endocrinology. 1958 Nov;63(5):619–628. doi: 10.1210/endo-63-5-619. [DOI] [PubMed] [Google Scholar]
- Chernoff H. N., Dukelow W. R. Decapacitation factor purification with lipid solvents. J Reprod Fertil. 1969 Feb;18(1):141–144. doi: 10.1530/jrf.0.0180141. [DOI] [PubMed] [Google Scholar]
- Clegg E. D., Foote R. H. Phospholipid composition of bovine sperm fractions, seminal plasma and cytoplasmic droplets. J Reprod Fertil. 1973 Aug;34(2):379–383. doi: 10.1530/jrf.0.0340379. [DOI] [PubMed] [Google Scholar]
- DAVIS J. S., ALDEN R. H. Hormonal influence on lipid metabolism of rat uterus. Anat Rec. 1959 Aug;134:725–737. doi: 10.1002/ar.1091340406. [DOI] [PubMed] [Google Scholar]
- Darin-Bennett A., White I. G. Influence of the cholesterol content of mammalian spermatozoa on susceptibility to cold-shock. Cryobiology. 1977 Aug;14(4):466–470. doi: 10.1016/0011-2240(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Davis B. K., Byrne R., Bedigian K. Studies on the mechanism of capacitation: albumin-mediated changes in plasma membrane lipids during in vitro incubation of rat sperm cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1546–1550. doi: 10.1073/pnas.77.3.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Byrne R., Hungund B. Studies on the mechanism of capacitation. II. Evidence for lipid transfer between plasma membrane of rat sperm and serum albumin during capacitation in vitro. Biochim Biophys Acta. 1979 Dec 12;558(3):257–266. doi: 10.1016/0005-2736(79)90260-8. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Decapacitation and recapacitation of rabbit spermatozoa treated with membrane vesicles from seminal plasma. J Reprod Fertil. 1974 Nov;41(1):241–244. doi: 10.1530/jrf.0.0410241. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Effect of calcium on motility and fertilization by rat spermatozoa in vitro. Proc Soc Exp Biol Med. 1978 Jan;157(1):54–56. doi: 10.3181/00379727-157-39989. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Inhibitory effect of synthetic phospholipid vesicles containing cholesterol on the fertilizing ability of rabbit spermatozoa. Proc Soc Exp Biol Med. 1976 Jun;152(2):257–261. doi: 10.3181/00379727-152-39374. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Weaver D. E., Clegg E. D. Diacyl, alkenyl, and alkyl ether phospholipids in ejaculated, in utero-, and in vitro-incubated porcine spermatozoa. J Lipid Res. 1980 Feb;21(2):223–228. [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Popják G. Mechanism of induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase in human leukocytes. J Biol Chem. 1977 Jan 25;252(2):644–651. [PubMed] [Google Scholar]
- Friend D. S. Freeze-fracture alterations in guinea pig sperm membranes preceding gamete fusion. Soc Gen Physiol Ser. 1980;34:153–165. [PubMed] [Google Scholar]
- Friend D. S., Orci L., Perrelet A., Yanagimachi R. Membrane particle changes attending the acrosome reaction in guinea pig spermatozoa. J Cell Biol. 1977 Aug;74(2):561–577. doi: 10.1083/jcb.74.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSWAMI A., KAR A. B., CHOWDHURY S. R. UTERINE LIPID METABOLISM IN MICE DURING THE OESTROUS CYCLE: EFFECT OF OVARIECTOMY AND REPLACEMENT THERAPY. J Reprod Fertil. 1963 Oct;6:287–295. doi: 10.1530/jrf.0.0060287. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Kopf G. S. The regulation of spermatozoa by calcium cyclic nucleotides. Adv Cyclic Nucleotide Res. 1980;13:251–306. [PubMed] [Google Scholar]
- Gordon M. Localization of phosphatase activity on the membranes of the mammalian sperm head. J Exp Zool. 1973 Jul;185(1):111–120. doi: 10.1002/jez.1401850111. [DOI] [PubMed] [Google Scholar]
- Grogan D. E., Mayer D. T., Sikes J. D. Quantitative differences in phospholipids of ejaculated spermatozoa and spermatozoa from three levels of the epididymis of the boar. J Reprod Fertil. 1966 Dec;12(3):431–436. doi: 10.1530/jrf.0.0120431. [DOI] [PubMed] [Google Scholar]
- Iritani A., Niwa K. Capacitation of bull spermatozoa and fertilization in vitro of cattle follicular oocytes matured in culture. J Reprod Fertil. 1977 May;50(1):119–121. doi: 10.1530/jrf.0.0500119. [DOI] [PubMed] [Google Scholar]
- Jain Y. C., Anand S. R. The lipids of buffalo spermatozoa and seminal plasma. J Reprod Fertil. 1976 Jul;47(2):255–260. doi: 10.1530/jrf.0.0470255. [DOI] [PubMed] [Google Scholar]
- Johnson L. A., Pursel V. G., Gerrits R. J. Total phospholipid and phospholipid fatty acids of ejaculated and epididymal semen and seminal vesicle fluids of boars. J Anim Sci. 1972 Aug;35(2):398–403. doi: 10.2527/jas1972.352398x. [DOI] [PubMed] [Google Scholar]
- KOMAREK R. J., PICKETT B. W., GIBSON E. W., JENSEN R. G. LIPIDS OF PORCINE SPERMATOZOA, SEMINAL PLASMA AND GEL. J Reprod Fertil. 1965 Apr;9:131–136. doi: 10.1530/jrf.0.0090131. [DOI] [PubMed] [Google Scholar]
- Katz D. F., Yanagimachi R., Dresdner R. D. Movement characteristics and power output of guinea-pig and hamster spermatozoa in relation to activation. J Reprod Fertil. 1978 Jan;52(1):167–172. doi: 10.1530/jrf.0.0520167. [DOI] [PubMed] [Google Scholar]
- Komarek R. J., Pickett B. W., Gibson E. W., Lanz R. N. Composition of lipids in stallion semen. J Reprod Fertil. 1965 Dec;10(3):337–342. doi: 10.1530/jrf.0.0100337. [DOI] [PubMed] [Google Scholar]
- Kulangara A. C. Maintenance of plasma-derived proteins at much lower concentrations in the uterine lumen of the rabbit: a kinetic study of passage. J Reprod Fertil. 1976 Jan;46(1):189–194. doi: 10.1530/jrf.0.0460189. [DOI] [PubMed] [Google Scholar]
- MATTNER P. E. CAPACITATION OF RAM SPERMATOZOA AND PENETRATION OF THE OVINE EGG. Nature. 1963 Aug 24;199:772–773. doi: 10.1038/199772a0. [DOI] [PubMed] [Google Scholar]
- Misiewicz L. Wpływ progesteronu i testosteronu na stezenie lipidów w płynie jmy macicy u szczurów. Ginekol Pol. 1968 Aug;39(8):859–862. [PubMed] [Google Scholar]
- Murdoch R. N., White I. G. The metabolism of labelled glucose by rabbit spermatozoa after incubation in utero. J Reprod Fertil. 1967 Oct;14(2):213–223. doi: 10.1530/jrf.0.0140213. [DOI] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- Niwa K., Chang M. C. Effects of sperm concentration on the capacitation of rat spermatozoa. J Exp Zool. 1974 Sep;189(3):353–356. doi: 10.1002/jez.1401890308. [DOI] [PubMed] [Google Scholar]
- OLIVER M. F., BOYD G. S. Changes in the plasma lipids during the menstrual cycle. Clin Sci. 1953 May;12(2):217–222. [PubMed] [Google Scholar]
- Papahadjopoulos D., Vail W. J., Newton C., Nir S., Jacobson K., Poste G., Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977 Mar 17;465(3):579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- Pinsker M. C., Robertson R. T. Chemistry of spermatozoa decapacitation factor. J Reprod Fertil. 1969 Feb;18(1):175–175. doi: 10.1530/jrf.0.0180175-a. [DOI] [PubMed] [Google Scholar]
- Poulos A., Darin-Bennett A., White I. G. The phospholipid-bound fatty acids and aldehydes of mammalian spermatozoa. Comp Biochem Physiol B. 1973 Nov 15;46(3):541–549. doi: 10.1016/0305-0491(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Poulos A., White I. G. The phospholipid composition of human spermatozoa and seminal plasma. J Reprod Fertil. 1973 Nov;35(2):265–272. doi: 10.1530/jrf.0.0350265. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., White I. G. Phospholipid and cholesterol content of epididymal and ejaculated ram spermatozoa and seminal plasma in relation to cold shock. Aust J Biol Sci. 1967 Dec;20(6):1205–1215. doi: 10.1071/bi9671205. [DOI] [PubMed] [Google Scholar]
- Scott T. W. Lipid metabolism of spermatozoa. J Reprod Fertil Suppl. 1973 Jul;18:65–76. [PubMed] [Google Scholar]
- Singh E. J. Oral contraceptives and human cervical mucus lipids. Am J Obstet Gynecol. 1975 Sep 15;123(2):128–132. doi: 10.1016/0002-9378(75)90515-3. [DOI] [PubMed] [Google Scholar]
- White I. G., Rodger J. C., Murdoch R. N., Williams W. L., Abney T. O. Effect of decapacitation factor on the oxygen uptake of rabbit spermatozoa recovered from the uterus. Experientia. 1975 Jan 15;31(1):80–81. doi: 10.1007/BF01924689. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R., Usui N. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res. 1974 Nov;89(1):161–174. doi: 10.1016/0014-4827(74)90199-2. [DOI] [PubMed] [Google Scholar]


