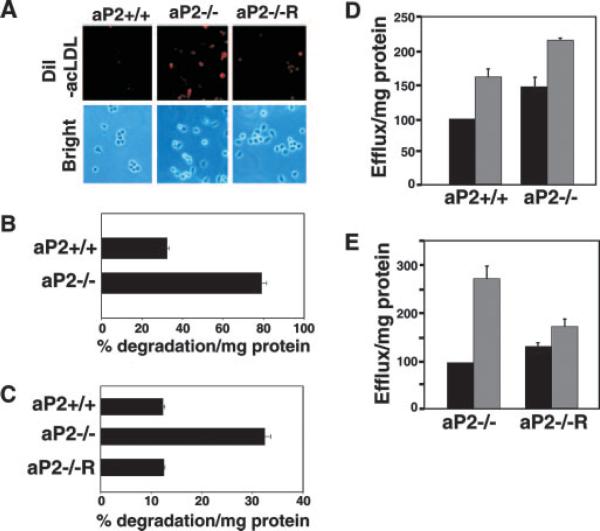
Fig. 1. aP2 deficiency accelerates cholesterol trafficking.
A, internalization of acLDL is enhanced by aP2 deficiency. aP2+/+, aP2−/−, and aP2−/−R macrophages (Brightfield) were treated with 10 μg/ml DiI-acLDL (red) for 4 h. B, aP2 deficiency enhances flux of cholesterol into macrophages. oxLDL internalization by thioglycollate-elicited macrophages was assessed by treatment with 125I-oxLDL in the presence or absence of excess unlabeled oxLDL for 24 h. Specific degradation is the percent uptake normalized to milligram of cellular protein ± S.E. C, stable expression of aP2 into aP2−/− macrophages (aP2−/−R) restores oxLDL uptake to aP2+/+ levels. Macrophage cell lines were treated the same as in B. D, cholesterol efflux from macrophages is increased by aP2 deficiency at base line and with induction. Thiogly-collate-elicited aP2+/+ and aP2−/− macrophages were loaded for 24 h with [3H]AcLDL, and cholesterol efflux to 0 (black bar) or 10 μg/ml (gray bar) apoA1 proceeded for 3 h. Percent efflux was normalized to cellular protein content and expressed as a percentage relative to aP2+/+ cultured with no apoA1 (100%) ± S.E. E, stable restoration of aP2 into aP2−/− macrophages reduces induced cholesterol efflux. Macrophage cell lines were treated as in D with the exception that 0 (black bar) or 15 μg/ml (gray bar) apoA1 was used.