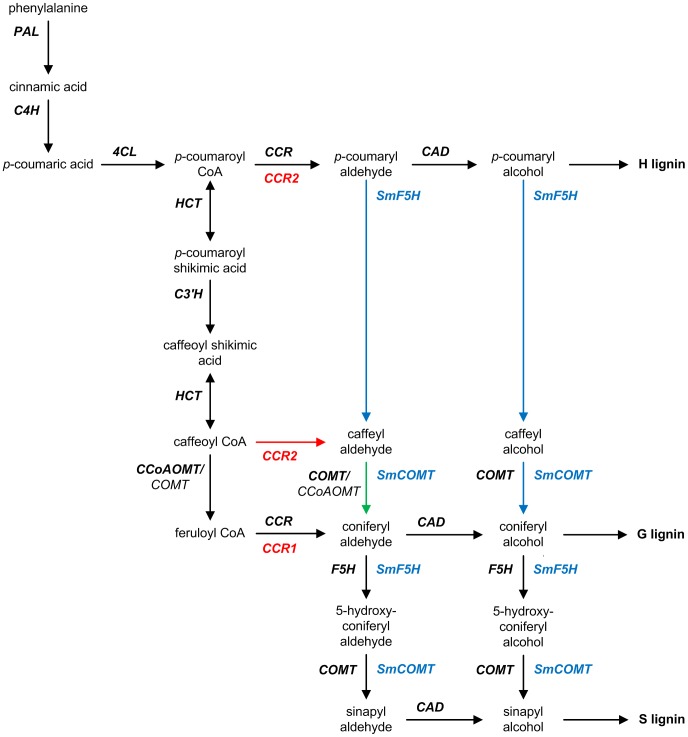
Figure 1. Generic pathway diagram of lignin biosynthesis with species-specific extensions.
The widely accepted generic pathway consists of black arrows. It leads to the biosynthesis of three hydroxycinnamyl alcohol monomers that in turn give rise to p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) subunits of lignin. Listed next to each reaction arrow are the catalyzing enzymes, which are highlighted in bold if considered major. The lycophyte Selaginella moellendorffi contains two bi-functional enzymes, SmF5H and SmCOMT, which are shown in blue. Co-expression of these two enzymes would permit S. moellendorffi to synthesize coniferyl and sinapyl alcohol directly from p-coumaryl aldehyde and p-coumaryl alcohol. By contrast, Medicago truncatula has two functionally distinct isoforms of CCR, which are shown in red. The green arrow that connects caffeyl aldehyde to coniferyl aldehyde denotes the only non-canonical reaction that is likely to be functional in both S. moellendorffi and M. truncatula. Abbreviations: CAD, cinnamyl alcohol dehydrogenase; CCoAOMT, caffeoyl CoA O-methyltransferase; CCR, cinnamoyl CoA reductase; C3′H, p-coumaroyl shikimate 3′-hydroxylase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumarate:CoA ligase; COMT, caffeic acid O-methyltransferase; F5H, ferulate 5-hydroxylase; HCT, hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase; PAL, L-phenylalanine ammonia-lyase.