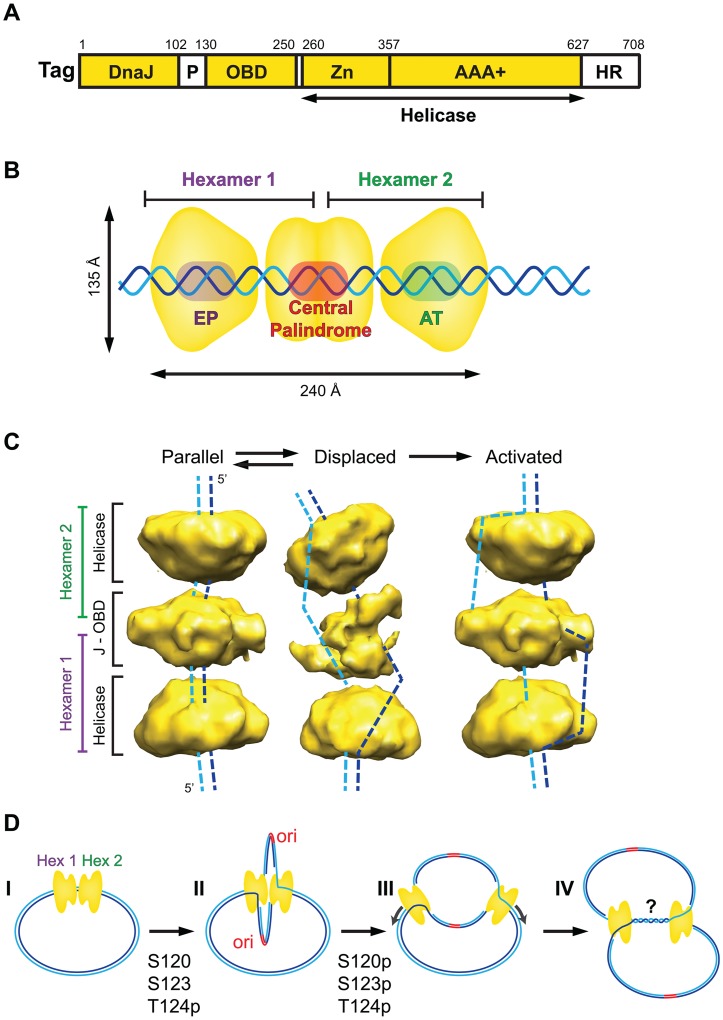
Figure 1. Assembly and activation of the SV40 pre-replication complex in vitro.
(A) Domain architecture of SV40 Tag. Three structured domains (yellow) (DnaJ chaperone domain, origin DNA binding domain [OBD], and helicase domain), composed of the zinc (Zn) and AAA+ ATPase sub-domains, are connected by flexible regions (white) (P, cluster of phosphorylated residues that regulates origin activation; HR, host range function). (B) Diagram of ADP-associated SV40 Tag double hexamer bound to the duplex SV40 core origin of DNA replication (EP, central palindrome, AT), with non-origin DNA protruding from the complex (adapted from [6]). (C) 3D cryo-electron microscopy reveals two conformations (parallel, displaced) of ADP-associated hypo-phosphorylated SV40 Tag double hexamer on SV40 origin DNA as in (B) (adapted from [6]). A hypothetical conformation for the activated double hexamer is shown at the right. Dashed lines suggest potential paths of the DNA strands through each protein conformation. (D) Stages of SV40 replication. I, Tag dodecamer assembled on duplex SV40 DNA as in (B); II, hypo-phosphorylated Tag dodecamer activated as in (C) unwinds DNA bidirectionally [13] and may assemble host proteins (not shown here) into two sister replisomes that interact physically through the central lobe of the Tag dodecamer; III, hyper-phosphorylation of Tag disrupts interactions between the hexamers [7], [8], releasing the replisomes to progress independently along the template chromatin; IV, replication forks converge slowly, accompanied by DNA decatenation, to complete replication, which may involve additional host proteins [3], [14], [18]–[21].