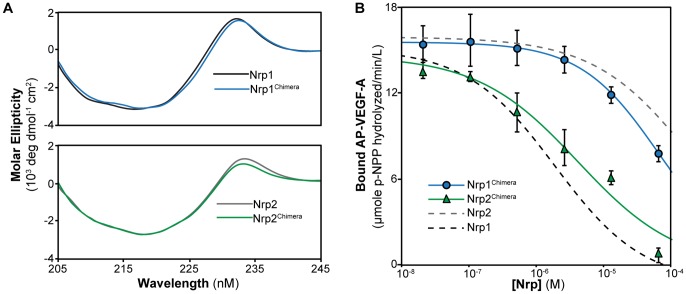
Figure 3. NrpChimera molecules exhibit reversed VEGF-A specificity.
(A) The secondary structure of WT Nrp and NrpChimera was assessed by CD. The overlapping spectra of NrpChimera with wild-type Nrp demonstrate that the incorporated mutations are not structurally deleterious. (B) Nrp1Chimera (blue line) and Nrp2Chimera (green line) were tested for their ability to selectively sequester AP-VEGF-A from Nrp1 adsorbed on affinity plates. The NrpChimera molecules show reversed VEGF-A specificity with Nrp1Chimera having a marked reduction in inhibitory potency (IC50 = 62 µM) and Nrp2Chimera exhibiting a significant gain in potency (IC50 = 3.9 µM). Wild-type Nrp1 (black dotted line, IC50 = 1.8 µM) and Nrp2 (grey dotted line, IC50≈310 µM) are shown for comparison (data from Figure 1). Experiments were performed in triplicate and reported as the mean ±1 S.D.