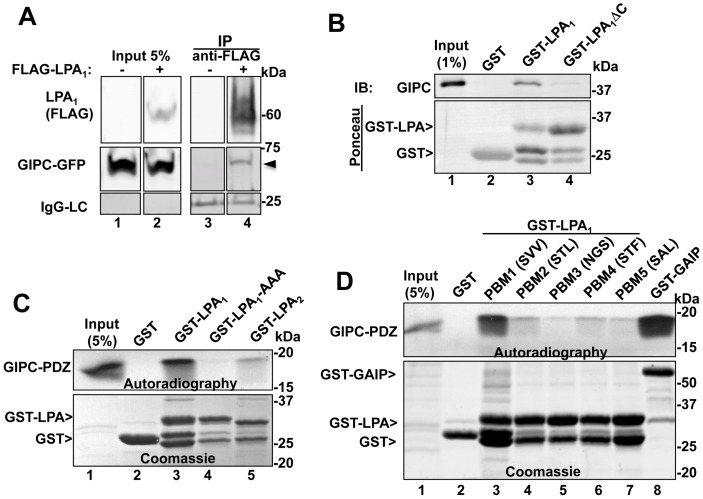
Figure 1. GIPC directly interacts with the C-terminal PDZ binding motif of LPA1 but not with other LPA receptors.
A, Endogenous GIPC and GIPC-GFP co-immunoprecipitate with FLAG- LPA1 from HEK cells expressing FLAG- LPA1 (arrowhead, lane 4) but not control HEK cells (lane 3). C-terminally tagged GIPC-GFP and N-terminally tagged FLAG-LPA1 were transiently coexpressed in HEK293 cells, and immunoprecipitation was carried out on cell lysates with mouse anti-FLAG IgG followed by immunoblotting with mouse anti-FLAG (LPA1) and rabbit anti-GIPC IgG. Lanes were cropped from a single exposure of a continuous membrane. The lower panel shows the amount of IgG light-chain (IgG-LC) in each IP. Lanes 1–2: Input showing the amounts of LPA1 and GIPC present in the lysates used for the IP. B, Upper panel: GIPC binds GST-LPA1 (GST fused to the cytoplasmic tail of mouse LPA1 (aa 311–364), lane 3) but not to GST alone (lane 2) or GST-LPA1ΔC (lacking the last three C-terminal amino acids, lane 4). Immobilized recombinant GST, GST-LPA1 and GST- LPA1ΔC were incubated 4–15 h with lysates from HEK293 cells transiently transfected with FLAG-GIPC. Proteins bound to immobilized fusion proteins were eluted with 2X sample buffer for SDS-PAGE and immunoblotted with anti-GIPC IgG. Lane 1: input, showing the amount of GIPC in 1% of the lysate used for the assay. Lower panel : Ponceau staining demonstrating the amount of GST proteins used in each assay. C, Upper panel: Autoradiography showing that in vitro translated, [35S]GIPC PDZ domain binds to GST-LPA1 (lane 3) but not to GST alone (lane 2), GST- LPA1AAA (last three amino acids mutated to alanine, lane 4), or GST-LPA2 (lane 5). GST fusion proteins were immobilized on glutathione-agarose beads as in “B” and incubated with in vitro translated [35S]Met-labeled, GIPC PDZ domain (aa 125–225). Bound proteins were separated by SDS-PAGE and detected by autoradiography. Lane 1: 5% of the in vitro translated protein. Lower panel : Coomassie Blue staining showing the GST proteins used for the assay. D, Upper panel: Autoradiography showing that in vitro translated, [35S] GIPC-PDZ interacts with the C-terminal PDZ binding motif of LPA1 (SVV, lane 3) and with GST-GAIP (lane 8, used as a positive control [7] but shows little or no interaction with GST alone (lane 2) or GST-LPA1 mutants in which the three C-terminal amino acids were modified to those of LPA2 (STL, lane 4), LPA3 (NGS, lane 5), LPA4 (STF, lane 6) or LPA5 (SAL, lane 7). Immobilized GST fusion proteins were incubated with in vitro translated [35S]Met-labeled GIPC PDZ and analyzed as in C. Lower panel: Coomassie Blue staining showing the amounts of GST proteins used.