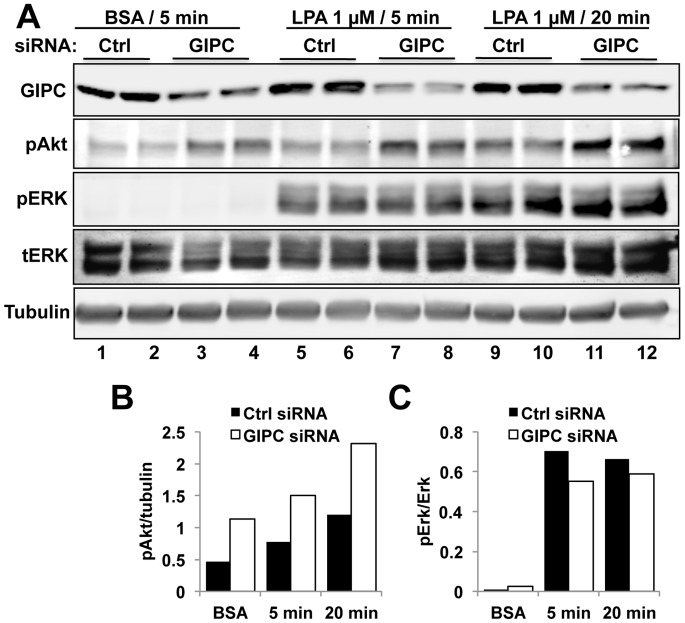
Figure 4. Akt phosphorylation is increased in GIPC depleted HEK-LPA cells.
A, In HEK- LPA1 cells transfected with control siRNA both Akt and ERK1/2 activation are enhanced after stimulation with LPA for 5 (lanes 5–6) or 20 min (lanes 9–10). GIPC depletion (GIPC siRNA) enhanced Akt phosphorylation (pAkt) at 5 min (lanes 7–8) and 20 min (lanes 11–12) after LPA stimulation. GIPC depletion also enhanced Akt signaling in the absence of ligand (lanes 1–4), possibly due to enhanced basal activity of LPA1. Erk phosphorylation (pERK) was not affected by GIPC depletion. HEK-LPA1 cells were transfected with control or GIPC siRNA, serum starved overnight, stimulated with 1 µM LPA or incubated with BSA alone for 5 or 20 min, lysed in RIPA buffer and analyzed by immunoblotting using phospho-Erk (pErk), total Erk (tErk), phospho-Akt (pAkt) and α-tubulin IgG. Each treatment was done in duplicate. α-tubulin was used as a loading control. In cells transfected with GIPC siRNA (lanes 3–4, 7–8, 11–12), GIPC expression is reduced 70–80% in cells transfected with control siRNA (Ctrl, lanes 1–2, 5–6, 9–10). B–C, Densitometric analysis of the immunoblots in A demonstrating that GIPC depletion (siRNA) leads to a two-fold increase in Akt phosphorylation (B) at both 5 and 20 min after LPA stimulation (B, P<0.05) but does not significantly affect Erk phosphorylation (C) compared to controls (Ctrl siRNA).