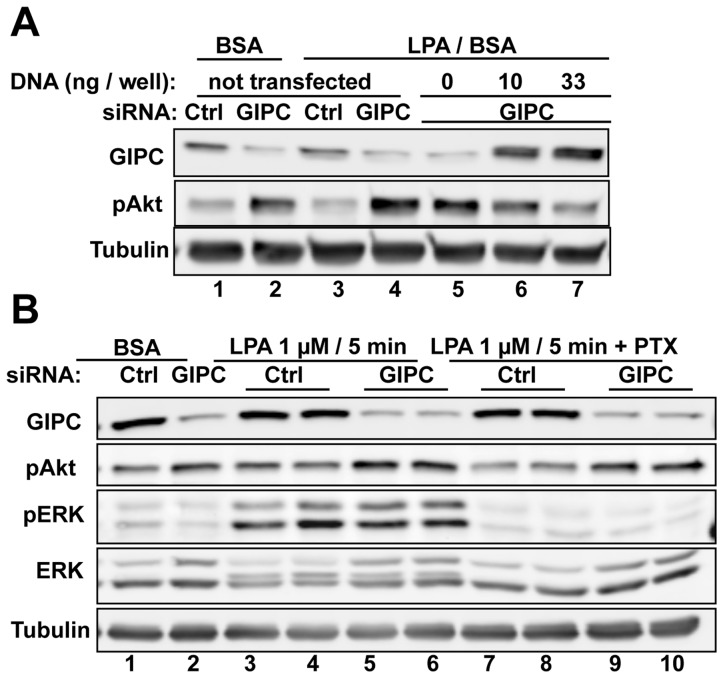
Figure 5. Enhancement of Akt activation following GIPC depletion is reversed by GIPC expression and is independent of Gαi signaling.
A, GIPC depleted HEK-LPA1 cells show reduced Akt phosphorylation after transfection of siRNA resistant GIPC DNA (lanes 6–7, middle panel) verifying that GIPC is responsible for the enhanced Akt phosphorylation seen after GIPC depletion. HEK-LPA1 cells were transfected with GIPC or control siRNA, and 12 h later they were transfected with siRNA-resistant GIPC DNA (0, 10, or 33 ng). After 24 h cells were serum starved overnight, stimulated with 1 µM LPA for 5 min, and cell lysates were immunoblotted for GIPC, pAkt and tubulin (used as loading control). B, Activation of Gαi is not required for the enhanced Akt phosphorylation seen after GIPC depletion. In GIPC-depleted cells PTX treatment (lanes 7–10) prevented LPA induced Erk phosphorylation (pErk) (which is Gαi dependent) but did not affect Akt phosphorylation (pAkt) compared with controls (lanes 3–6). 36 h after siRNA transfection, HEK-LPA1 cells were cultured for another 12 h in serum-free media in the presence or absence of PTX and then stimulated for 5 min with 1 µM LPA (in 0.1% BSA, lanes 3–10) or incubated in BSA alone (0.1%) for 5 min (lanes 1–2).