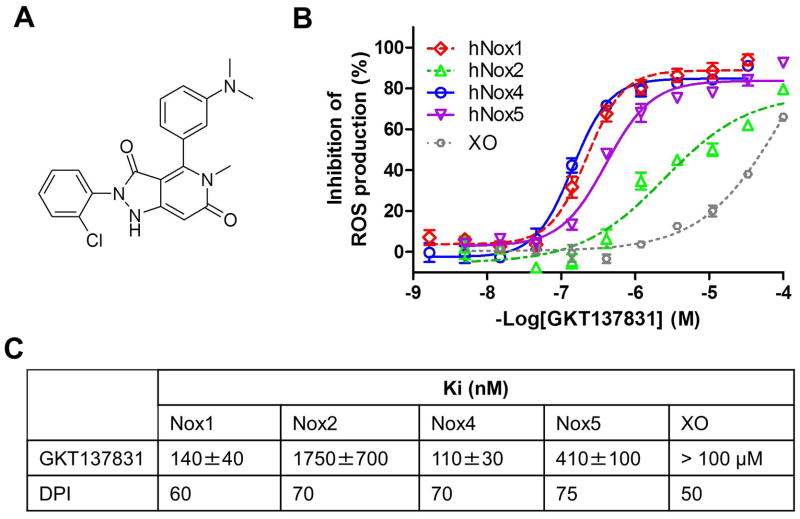
Fig. 2. Pharmacological profile of GKT137831, a dual Nox1/Nox4 inhibitor.
(A) Chemical structure of the dual Nox1/Nox4 inhibitor GKT137831. (B) Inhibition of Nox-dependent ROS production by GKT137831: concentration-response curves of GKT137831 on membranes prepared from cells specifically overexpressing hNox1 (◇), hNox2 (△), hNox4 (○), hNox5 (▽) and on Xanthine Oxidase (XO) (○). Km of NADPH for hNox1, hNox2, hNox4 and hNox5 and was 70±10mM, 16±3mM, 120±20mM and 70 ±10mM respectively and Km of Xanthine for XO was 6±1mM. Results are from one experiment performed in triplicate, representative of at least three performed. Values are means±SEM. (C) Inhibition constants (Ki) of GKT137831 and DPI on hNox1, hNox2, hNox4, hNox5 and XO.