Abstract
BACKGROUND:
Polycystic ovarian syndrome is a common endocrine disorder of reproductive age women. Many controlled ovarian stimulation (COS) strategies have been offered for the treatment of patients with PCOS undergoing in vitro fertilization, but the optimal protocol is still a controversy. There is no compelling evidence for the advantage of one stimulation protocol over the other.
MATERIALS AND METHODS:
This is a single-center prospective controlled study comparing long agonist and antagonist protocol in PCOS women.
RESULTS:
There was no significant difference in live birth rate and clinical pregnancy rate. Rate of ovarian hyperstimulation syndrome was significantly higher in the agonist group. Number of oocytes retrieved, number of follicles and peak estradiol levels were significantly more in the agonist group.
CONCLUSION:
The GnRH antagonist protocol is an equally effective but safer protocol in PCOS patients compared with the long agonist protocol.
KEY WORDS: COS, IVF outcome, PCOS
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a common endocrinopathy that affects 5–10% of women of the reproductive age group.[1] The clinical presentation varies from eumenorrhea and a sonographic picture of polycystic ovaries with subtle phenotypic abnormalities or signs of hyperandrogenism to advanced polycystic ovarian syndrome and its associated long-term sequelae.[2] Although the criteria for diagnosis of PCOS has been universally accepted as per the Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group 2004,[3] the optimal infertility treatment for PCOS is still a controversy. These controversies surrounding the treatment have led to the recently published ESHRE/ASRM consensus that adhered to the therapeutic challenges raised in women with infertility and PCOS, the various treatments available and their efficacy as well as safety.[4]
In vitro fertilization (IVF) remains a reasonable option in PCOS women who are refractory to conventional infertility treatment modalities or who have coexisting infertility factors.[3] Many controlled ovarian stimulation (COS) strategies have been offered for the treatment of patients with PCOS undergoing IVF. However, there is no compelling evidence for the advantage of one stimulation protocol over the other.[3] The ESHRE/ASRM consensus document has recently stressed the need to perform further randomized controlled trials (RCTs) comparing FSH stimulation protocols with the use of GnRH agonist versus GnRH antagonists.[3] PCOS patients undergoing COS have a high risk of developing ovarian hyperstimulation syndrome (OHSS), a serious iatrogenic complication of ovarian stimulation.[4] Gonadotropin releasing hormone antagonists have been shown to offer an advantage over standard long agonist protocol in terms of decreasing incidence of OHSS, short duration of treatment, lower cost, lesser dose of gonadotropins required and being more patient friendly. Although there are some RCTs comparing GnRH agonists versus antagonists in the PCOS population, there is still a lack of consensus as to which protocol is better.[5–7]
The aim of the present study was to compare the GnRH agonist long protocol with the flexible GnRH antagonist protocol in infertile PCOS women undergoing COS in terms of clinical pregnancy rate (CPR), with special reference to the incidence of OHSS in the Indian population.
MATERIALS AND METHODS
Patient population and study design
This was a single-center prospective controlled study conducted from February 2009 to June 2010. Patients were assigned to two groups on Day 2 of the menstrual cycle: 60 cases received standard GnRH agonist long protocol and 40 were allocated to the GnRH antagonist protocol. No randomization was done. Inclusion criteria for the study were: PCOS as defined by the Rotterdam 2003 consensus, i.e. presence of two of the following three features: presence of oligo- and/ or anovulation, clinical and/or biochemical signs of hyperandrogenism, polycystic ovaries and exclusion of other endocrinopathies,[3] age 18–35 years, body mass index of 18–30 kg/m2, baseline Follicle stimulating hormone <10 IU/L, normal uterine cavity as assessed by hysteroscopy and no evidence of thyroid or prolactin dysfunction. Patients falling out of the above criteria, with presence of congenital uterine malformations, Asherman syndrome, genital tuberculosis, surgical retrieved sperms, hydrosalpinx and those showing poor response in previous IVF cycles were excluded from the study. Written informed consent was obtained from all cases and the study was approved by the Institutional Ethics Board.
Study methodology
All patients underwent baseline transvaginal sonography on Day 2/3 of the menstrual cycle to check for antral follicle count and endometrial thickness and to rule out the presence of ovarian cyst. All patients received oral contraceptive pills for 21 days prior to the treatment cycle. Patients were assigned to the agonist or antagonist group non-randomly. In the agonist group, treatment was started from Day 21 of the menstrual cycle with inj. Leupride acetate 0.5 mg (Sun Pharmaceutical Ind Ltd, Mumbai) subcutaneously once daily till downregulation was achieved/Day 2 of menstrual cycle (defined as serum estradiol <50 pg/mL, endometrial thickness <5 mm, no cyst in the ovaries, serum Leutinising hormone <2.0 IU/L). Once downregulation was achieved, the inj. Leupride dosage reduced to 0.2 mg daily and recombinant FSH (inj. Gonal-f, Merck Serono Specialities Pvt. Ltd., Italy) was started. The starting dose of recombinant FSH was 75–150 IU. The dose was adjusted after 4 days of stimulation depending on the ovarian response, assessed by transvaginal scan (using 7.5 MHz vaginal probe, Voluson 730 pro, GE Healthcare, Milwaukee, Winconsin USA) and serum estradiol levels. Human menopausal gonadotropin (Ovugraf HP®, VHB Life Sciences Ltd., Mumbai, India) was added in the later days of stimulation on an individual basis according to the physician's discretion. Follicular growth was monitored by serial ultrasonography. The dose of FSH and HMG was adjusted according to serum estradiol levels and dynamics of ovarian follicular growth.
In the flexible antagonist protocol, inj. recombinant FSH (inj. Gonal-f, Merck Serono Specialities Pvt. Ltd.) was started on Day 2 of the cycle (75–150 IU daily). GnRH antagonist inj. Orgalutran 0.25 mg s/c (inj. Ganirelix 0.25 mg, Organon India Ltd, Kolkata. Ltd.) was started when the lead follicle reached a diameter of 14 mm and/or the estradiol levels were >400 pg/mL. Inj. HMG was added in the later part of the cycle on an individual basis. Treatment with rFSH and antagonist was continued till the day of final oocyte maturation trigger.
When three or more follicles of size 18 mm or more were seen, final oocyte maturation trigger was given with inj. hCG 5000 IU intramuscular (inj. Ovumax-HP, VHB Life Sciences Ltd.). Oocyte retrieval was performed 35 h after the hCG injection by transvaginal-guided single-lumen needle aspiration. Oocyte assessment was performed by standard morphology criteria proposed by Lin et al.,[8] and nuclear maturity assessment was performed in cases subjected to intracytoplasmic sperm injection (ICSI). Conventional IVF or ICSI was performed depending on the semen parameters and previous fertilization history. Culture media used was vitrolife (Vitrolife Sweden AB, Goteborg, Sweden). Fertilization was defined as presence of pronuclei 16–18 h post-insemination/injection. Embryo grading was done by standard morphology assessment according to the modified Veecks’ scoring.[9] Embryo Transfer was done on Day 3/5 following oocyte retrieval. All patients were prescribed 600 mg of micronized progesterone as luteal phase support for 2 weeks. Serum estradiol, LH and progesterone levels were measured on the day of hCG administration and compared in the two groups. Measurement of estradiol, progesterone, LH, FSH and βhCG was done by fully automated electro-chemiluminscence technology (Roche Cobas e411 analyzer, HITACHI, Tokyo, Japan). βhCG >50 IU/L or gestational sac on Trans-vaginal sonography 2 weeks after embryo transfer was considered as positive for pregnancy.
Grading of OHSS
Classification was based on the modified classification system based on combined criteria reported by Golan et al., Navot et al.[10,11].
Grade one included patients with mild OHSS who had abdominal bloatedness but did not need admission or treatment because of ovarian stimulation. Grade two included patients who had sonographic evidence of ascites with abdominal pain and/or vomiting but were not hospitalized and required treatment on outpatient basis. Grade three included women who were hospitalized either because of critical OHSS or fulfilled one or more of the criteria for hospitalization described by the Practice committee of ASRM 2004.[12]
Comparison was made in two groups regarding cases of moderate–severe OHSS.
Outcome measures
The primary outcome measure was live birth rate (defined as number of deliveries that resulted in at least one live born baby per ET) and clinical pregnancy rate (defined as presence of gestational sac with fetal heart rate at 6–7 weeks gestation per ET). Secondary outcome measures were rate of OHSS, days of stimulation, dose of gonadotropins required, peak estradiol levels on day of hCG, fertilization rate, implantation rate, multiple pregnancy rate and miscarriage rate. Implantation rate was calculated by dividing the number of gestational sacs seen on transvaginal sonography by number of embryos transferred. Ectopic pregnancy was described as presence of extrauterine gestational sac and βhCG >1000 IU/L in the absence of intrauterine gestational sac. Miscarriage was defined as discontinuation/failure to grow a pregnancy before 12 weeks of gestation. Embryology details included the mean number of mature oocytes retrieved per patient, number of lead follicles on day of hCG (>16 mm size), fertilization rate, cleavage rate, endometrial thickness on day of embryo transfer and number of cases where embryos were frozen. Hormonal profile included serum estradiol, LH and progesterone levels on day of hCG.
Statistical methods
Descriptive statistical analysis has been carried out in the present study. Results on continuous measurements are presented as mean ± SD (min–max) and results on categorical measurements are presented as number (%). Significance is assessed at the 5% level of significance. Student's t test (two-tailed, independent) has been used to determine the significance of the study parameters on a continuous scale between the two groups [intergroup analysis]) on metric parameters and Chi-square/Fisher Exact test has been used to find the significance of the study parameters on a categorical scale between two or more groups. P-value of <0.05 was taken as significant.
Statistical software
The statistical software, namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1, Systat 12.0 and R environment ver.2.11.1, were used for the analysis of the data and Microsoft Word and Excel have been used to generate graphs, tables, etc.
Although the sample size is small looking at the baseline pregnancy rate for PCOS patients being 35–40%. However, we undertook the study to compare the response of Indian PCOS patients to GnRH antagonists and agonists.
RESULTS
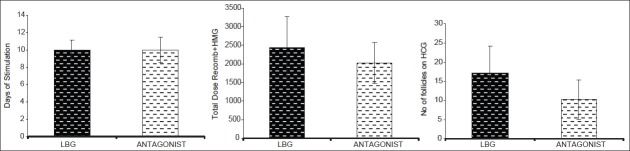
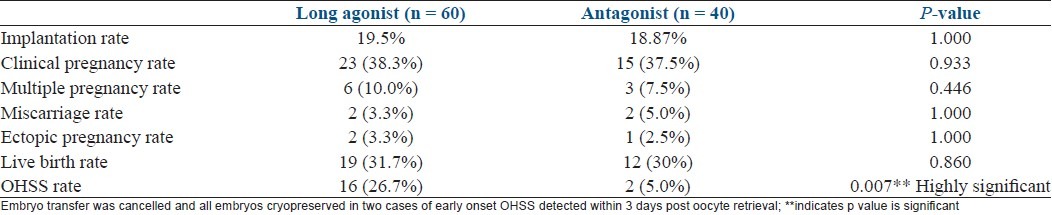
Baseline characteristics are depicted in Table 1. There was no significant difference in the baseline parameters in the two groups. Table 2 shows comparasion of two groups regarding stimulation characteristics. Embryology parameters are depicted in Table 3 and Figure 1. OHSS rate was significantly more in agonist group [Table 4].
Table 1.
Baseline parameters of patients in the agonist and antagonist groups (n = 100)
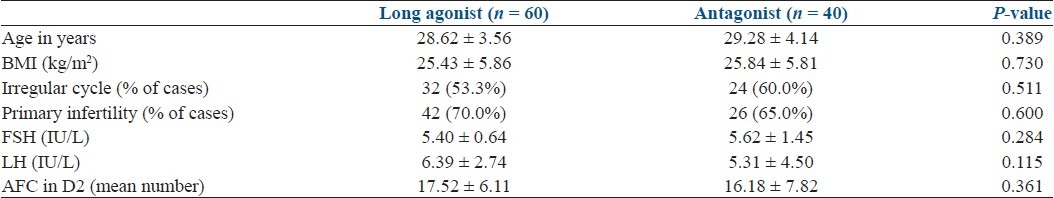
Table 2.
Ovarian stimulation characteristics and hormonal profile on the day of hCG (n = 100)
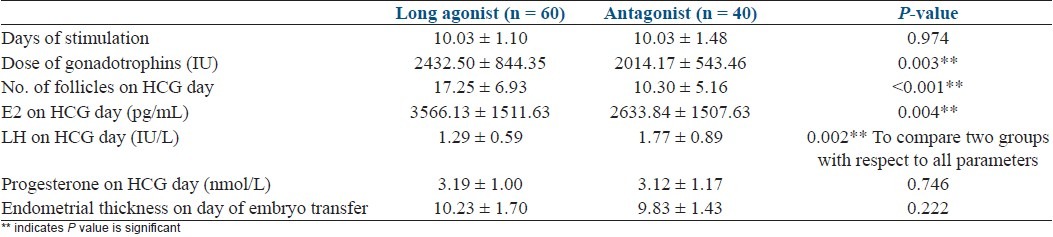
Table 3.
Embryology data in the agonist and antagonist groups (n = 100)
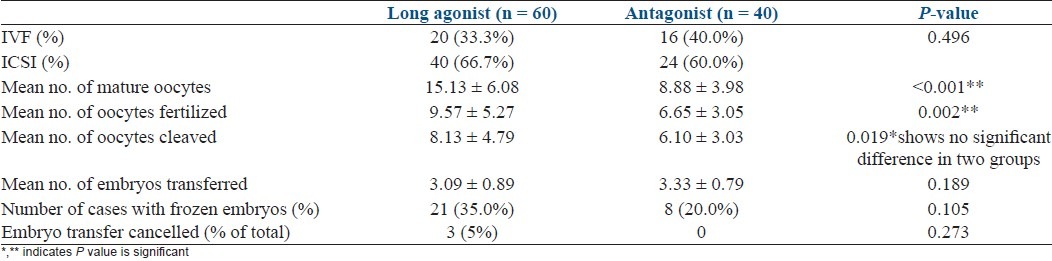
Figure 1.
Stimulation characteristics of two groups
Table 4.
Pregnancy outcome and OHSS rate in the agonist and antagonist groups
DISCUSSION
For more than 20 years, GnRH agonists have been the “gold standard” protocol in COS. The vast majority of IVF treatment cycles are still performed using the GnRH agonist long protocol. GnRH antagonists, in contrast, are more often used as second-line agents in patients who are poor responders, in the elderly and in the ones with previous IVF failures.
The present study showed that clinical pregnancy rate and live birth rate were not significantly different in the agonist versus the antagonist groups. Fertilization rate and clearage rate did not show any significant difference in the two groups. But, the OHSS rate was significantly lower in the antagonist protocol. Severe OHSS is a life-threatening complication of ovulation induction and should be an important consideration when deciding the treatment plan for PCOS patients. Although COS using the long agonist protocol was shown to be associated with a significantly higher clinical pregnancy rate than the GnRH antagonist protocol, it resulted in an increased incidence of severe OHSS.[13,14] Therefore, in patients with a high risk of OHSS, GnRH antagonist should be the preferred protocol. It enables the use of GnRH agonist instead of hCG as ovulation trigger, which markedly decreased the incidence of OHSS.[2] Therefore, use of GnRH antagonists in PCOS patients results in a safer way of performing ovarian stimulation for IVF.
The reason for the higher incidence of OHSS in the agonist group may have been more number of follicles and oocytes retrieved and, additionally, more number of intermediate-sized follicles, leading to high peak estradiol levels. There was no significant difference in days of stimulation in the two groups, but dose of gonadotrophins required was significantly higher in the long agonist group.
In the present study, it was shown that the flexible GnRH antagonist protocol was associated with a significantly lower probability of moderate–severe OHSS (consequently the need for hospitalization) compared with the long agonist protocol. These results are in corroboration with previous studies in the general population (Kolibianakis et al., Al- Inany et al., Cochrane).[13–15] This difference in incidence of OHSS is in accordance with more number of oocytes retrieved and higher peak estradiol levels in the agonist group. Taking into account the severity and importance of this purely iatrogenic complication, the reduction of OHSS incidence should be welcomed.
According to the Cochrane 2011 review of 45 RCTs, use of antagonist compared with long GnRH agonist protocols has been shown to be associated with a large reduction in OHSS, and there was no evidence of a difference in live birth rates. When only the women with PCOS were compared, there was no significant difference in the ongoing pregnancy rate (7 RCTs; OR 0.91, 95% CI 0.67–1.22; P = 0.94; I2 = 0%). Regarding the safety, a GnRH antagonist significantly reduced the incidence of OHSS by 50%. In addition, with GnRH antagonist treatment, the chance of cancellation or coasting due to high risk to develop OHSS was only 53% of that with the GnRH agonist treatment. The corresponding number needed to harm (NNH) was 25 (95% CI 19–36), with an absolute risk reduction of 4% (95% CI 2.79–5.13). This means that for every 25 women undergoing downregulation by an agonist, you would expect one more case of severe OHSS. In addition, the cancellation rate due to the high risk of developing OHSS was significantly higher in the GnRH agonist group. This indicates that the difference would be highly significant without cancellation, suggesting that GnRH antagonist is safer than GnRH agonist. Therefore, in patients at high risk of OHSS, the GnRH antagonist should be the preferred protocol during their first IVF attempt, because it enables the use of GnRH agonist, instead of hCG, to trigger ovulation, with the consequent elimination of severe OHSS. These benefits would justify a change from the standard long agonist protocol to antagonist regimens.[15]
Although more number of embryos were available and the cryopreservation rate was higher in the agonist group, still, there was no significant difference in cumulative clinical pregnancy rate and live birth rate. Different studies have shown variable results regarding pregnancy rates.[1,2,6,7] Results of the recent Cochrane review have shown that GnRH antagonists lead to similar pregnancy outcome but a markedly lower incidence of severe OHSS. In the present study, there was no significant difference in the miscarriage rates.[15]
The meta-analysis of Griesinger et al. compared agonist and antagonist protocol in a total of 305 patients with PCOS, and included four studies. In agreement with the results of the present study, pregnancy rates were not significantly different in the agonist and antagonist groups. But, while analyzing the patient at high risk of OHSS, the incidence of severe OHSS was significantly lower in the antagonist group.[6] Similar results have been shown by Ragini et al.[5]
In the present study, cycle cancellation rate due to OHSS was 5% in the agonist group, and there was no cancellation in the antagonist protocol. In the last Cochrane update, in the antagonist protocol, the risk of cycle cancellation or coasting due to high risk of developing OHSS was only 53% of that of the agonist protocol.[15]
The other main advantage of antagonist is that they are more patient friendly. Duration of treatment is short by at least 14 days in the antagonist, and dose of gonadotrophins administered may be low. Although this might not lead to direct reduction in the cost of treatment, but, if we take into consideration the cost of treatment per pregnancy including the cost of hospitalization due to OHSS, number of working hours lost due to prolonged treatment and inconvenience of multiple injections for more days, the final cost may be higher in the agonist protocol.[15,16] Although there are no studies on economic comparison in the two groups, according to the Cochrane review 2011, significant reduction in the incidence of severe OHSS in the antagonist group could have a direct impact on the reduction of cost of cycle.[15]
Additionally, there is no risk of withdrawal symptoms, risk of cyst formation and accidental administration of GnRH analogues during early pregnancy.[17,18] Today, there is an eager desire to shift to more patient-friendly mild ovarian stimulation protocols globally in which use of GnRH antagonists may be a suitable solution.[19]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lainas TG, Ioannis S, Zorzovillis IZ, Petsas GK, Lainas GT, Alexopoulou E, et al. Flexible GnRH antagonist protocol versus GnRH agonist long protocol in patients with polycystic ovary syndrome treated for IVF: A prospective randomized controlled trial (RCT) Hum Reprod. 2010;25:683–9. doi: 10.1093/humrep/dep436. [DOI] [PubMed] [Google Scholar]
- 2.Orvieto R, Meltcer S, Homburg R, Nahum R, Rabinson J, Ashkenazi J. What is the preferred GnRH analogue for polycystic ovary syndrome patients undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2009;91(4 Suppl):1466–8. doi: 10.1016/j.fertnstert.2008.07.1711. [DOI] [PubMed] [Google Scholar]
- 3.Rotterdam ESHRE/ASRM-sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Thessaloniki ESHRE/ ASRM- sponsored PCOS Consensus Workshop Group. Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril. 2008;89:505–22. doi: 10.1016/j.fertnstert.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Ragni G, Vegetti W, Riccaboni A, Engl B, Brigante C, Crosignani PG. Comparison of GnRH agonists and antagonists in assisted reproduction cycles of patients at high risk of ovarian hyperstimulation syndrome. Hum Reprod. 2005;20:2421–5. doi: 10.1093/humrep/dei074. [DOI] [PubMed] [Google Scholar]
- 6.Griesinger G, Diedrich K, Tarlatzis BC, Kolibianakis EM. GnRH-antagonists in ovarian stimulation for IVF in patients with poor response to gonadotropins, polycystic ovary syndrome, and risk of ovarian hyperstimulation: A meta-analysis. Reprod Biomed Online. 2006;13:628–38. doi: 10.1016/s1472-6483(10)60652-9. [DOI] [PubMed] [Google Scholar]
- 7.Tarlatzis BC, Kolibianakis EM. GnRH agonists vs antagonists. Best Pract Res Clin Obstet Gynaecol. 2007;21:57–65. doi: 10.1016/j.bpobgyn.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Lin YC, Chang SY, Lan KC, Huang HW, Chang CY, Tsai MY, et al. Human oocyte maturity in vivo determines the outcome of the blastocyst development in vitro. J Assist Reprod Genet. 2003;20:506–12. doi: 10.1023/B:JARG.0000013651.37866.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veek L. An Atlas of human gametes and conceptuses. London: Parthenon; 1999. p. 215. [Google Scholar]
- 10.Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E. Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv. 1989;44:430–40. doi: 10.1097/00006254-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Navot D, Bergh P, Laufer N. Ovarian hyperstimulation syndrome in novel reproductive technologies: prevention and treatment. Fertil Steril. 1992;58:249–61. doi: 10.1016/s0015-0282(16)55188-7. [DOI] [PubMed] [Google Scholar]
- 12.Ovarian hyperstimulation syndrome. Educational Bulletin, The Practice Committee of the American Society for Reproductive Medicine. Fertil Steril. 2008;90(Suppl 3):S188–93. doi: 10.1016/j.fertnstert.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Kolibianakis EM, Collins J, Tarlatzis BC, Devroey P, Diedrich K, Griesinger G. Among patients treated for IVF with gonadotrophins and GnRH analogues, is the probability of live birth dependent on the type of analogue used.? A systematic review and meta-analysis. Hum Reprod Update. 2006;12:651–71. doi: 10.1093/humupd/dml038. [DOI] [PubMed] [Google Scholar]
- 14.Al-Inany HG, Abou-Setta AM, Aboulghar M. Gonadotrophin-releasing hormone antagonists for assisted conception. Cochrane Database Syst Rev. 2006;3:CD001750. doi: 10.1002/14651858.CD001750.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Al-Inany HG, Youssef MA, Aboulghar M, Broekmans F, Sterrenburg M, Smit J, et al. Gonadotrophin releasing hormone antagonists for assisted reproductive technology. Cochrane Database Syst Rev. 2011;(5):CD001750. doi: 10.1002/14651858.CD001750.pub3. [DOI] [PubMed] [Google Scholar]
- 16.Ashrafi M, Moini A, Mohammadzadeh A, Ezabadi Z, Zafarani F, Baghestani AR, et al. A comparative study of GnRH antagonist and GnRH agonist in PCO patients undergoing IVF/ICSI cycles. Iranian J Reprod Med. 2005;3:14–8. [Google Scholar]
- 17.Felberbaum R. Gonadotropin-releasing hormone antagonists: Will they replace agonists? Reprod Biomed Online. 2002;6:43–53. doi: 10.1016/s1472-6483(10)62054-8. [DOI] [PubMed] [Google Scholar]
- 18.Reh A, Lewis Krey MD, Noyes N. Are gonadotropin-releasing hormone agonists loosing popularity. Current trends at a large fertility centre? Fertil Steril. 2010;93:101–8. doi: 10.1016/j.fertnstert.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Fauser BC, Nargund G, Andersen AN, Norman R, Tarlatzis B, Boivin J, et al. Mild ovarian stimulation for IVF: 10 years later. Hum Reprod. 2010;25:2678–84. doi: 10.1093/humrep/deq247. [DOI] [PubMed] [Google Scholar]