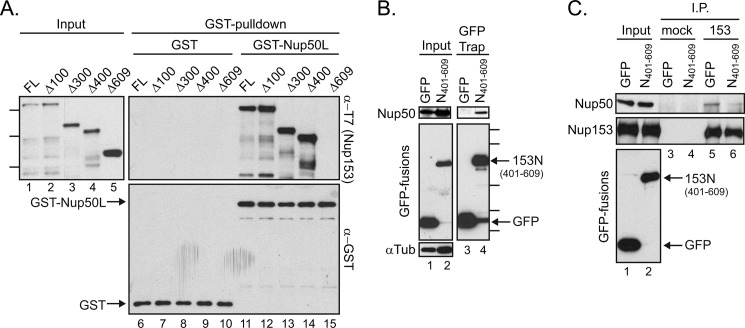
FIGURE 4.
Amino acids 401–609 within Nup153 are necessary and sufficient for contact with Nup50. A, bacterial lysates containing a panel of N-terminally truncated, recombinantly expressed Nup153 constructs were incubated with GST (lanes 6–10) or GST-Nup50L (lanes 11–15), followed by affinity purification on glutathione-Sepharose beads. Co-purifying Nup153-derived proteins were tracked by immunoblotting with the anti-T7 antibody (left panel and upper right panel), and recovery of GST proteins themselves was monitored by immunoblotting with anti-GST antibody (lower right panel). Molecular mass markers indicated are 170, 130, and 70 kDa. FL, full-length. B, GFP was recovered from lysates of cells expressing GFP alone or a GFP fusion with amino acids 401–609 of Nup153. Recovery of any co-isolated Nup50 (upper panels) along with each GFP protein (middle left panel and lower right panel) was tracked by immunoblotting. The levels of α-tubulin (αTub) were tracked to ensure equivalent loading of samples (lower left panel). Molecular mass markers indicated are 100, 70, 40, 35, and 25 kDa. C, Nup153 was immunoprecipitated (I.P.) from lysates of cells expressing GFP or GFP-Nup153(401–609) (lanes 5 and 6); material isolated with equivalent levels of protein A-Sepharose beads alone was run alongside (lanes 3 and 4). The precipitated material was immunoblotted for the presence of Nup153 and Nup50. For comparison, samples of the input material are shown (lanes 1 and 2).