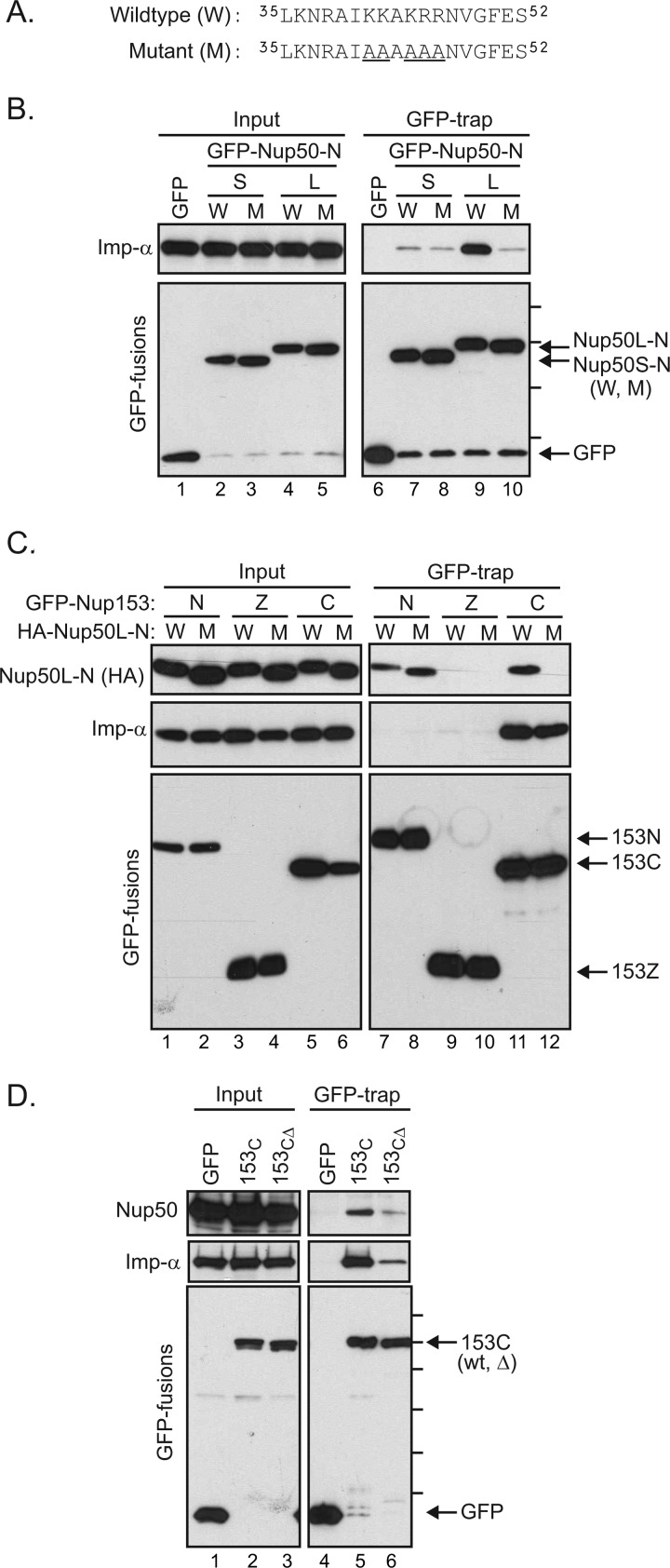
FIGURE 6.
Importin α mediates an interaction between the C-terminal tail of Nup153 and Nup50. A, the sequence context of mutations (underlined) used to test the role of the N-terminal region of Nup50 (9). Mutations were made in HA-tagged Nup50 N-terminal domain constructs. B, GFP fusions with the Nup50 N-terminal domain from the short (S) and long (L) isoforms in either the wild-type (W) or mutant (M) form were expressed. Material recovered by GFP-Trap was then immunoblotted to detect association of importin α (Imp-α). The molecular mass markers indicated are 70, 55, 40, and 30 kDa. C, following coexpression of Nup153 domain constructs (N, Z, and C) fused to GFP along with HA-tagged Nup50L-N in either the wild-type or mutant form, GFP proteins were recovered from cell lysates. GFP proteins, as well as co-isolating Nup50-N and endogenous importin α, were tracked by immunoblotting as indicated (inputs are 2% for HA-Nup50-N, 4% for importin α, and 8% for GFP). D, GFP fusion proteins were recovered from lysates of cells expressing GFP alone, a GFP fusion with the Nup153 C-terminal region, or a GFP fusion with a truncated version of the Nup153 C-terminal domain lacking the terminal 18 amino acids, previously defined as an importin α-binding motif. Recovery of endogenous Nup50 and importin α, along with each GFP protein, was tracked by immunoblotting as indicated (inputs are 6% for Nup50, 16% importin α, and 8% for GFP). Molecular mass markers indicated are 130, 100, 70, 55, 40, and 35 kDa.