FIGURE 4.
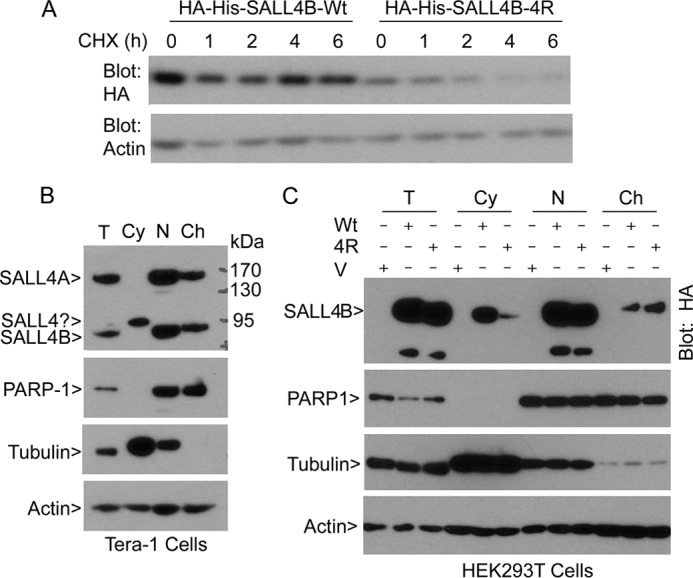
Sumoylation is important for the stability and subcellular localization of SALL4B. A, HEK293T cells transfected with a plasmid expressing HA-His-SALL4B (Wt) or HA-His-SALL4B mutant (4R) for 24 h were treated with cycloheximide (CHX) for various times as indicated. At the end of the treatment, cells were collected and lysed and approximately equal amounts of cell lysates were blotted with the antibody to HA and β-actin, respectively. B, cytoplasmic, nucleoplasmic, and chromatin fractions of Tera-1 cells were blotted with antibodies to SALL4, PARP-1, α-tubulin, and β-actin. Total cell lysates were also analyzed as control. C, HEK293T cells were transfected with the wild-type SALL4B (Wt) expression plasmid, its 4R mutant (4R), or vehicle (V) for 48 h, after which equal amounts of protein lysates of cytoplasmic, nucleoplasmic, and chromatin fractions were blotted with antibodies to HA, PARP-1, α-tubulin, and β-actin.
